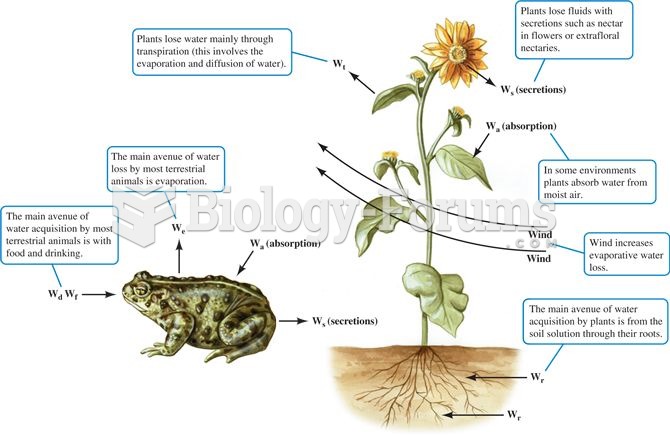
|
|
|
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Illicit drug use costs the United States approximately $181 billion every year.
The FDA recognizes 118 routes of administration.
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).