|
|
|
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Elderly adults are at greatest risk of stroke and myocardial infarction and have the most to gain from prophylaxis. Patients ages 60 to 80 years with blood pressures above 160/90 mm Hg should benefit from antihypertensive treatment.
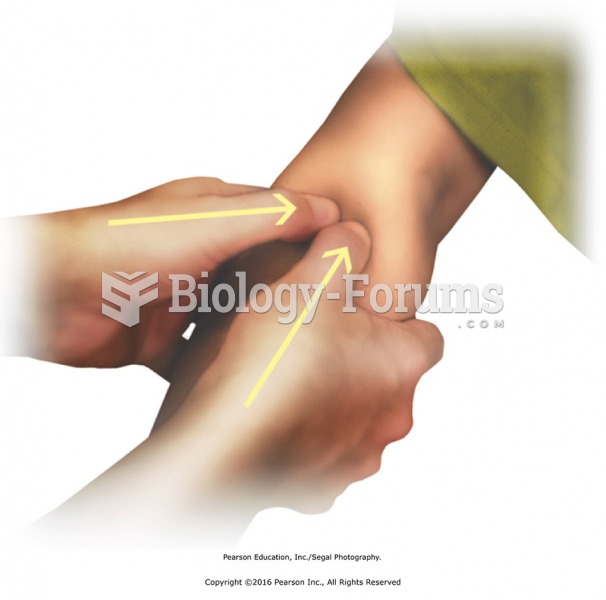
 Effleurage to transition to lower leg-distal to proximal. Apply effleurage with moderate pressure to ...
Effleurage to transition to lower leg-distal to proximal. Apply effleurage with moderate pressure to ...
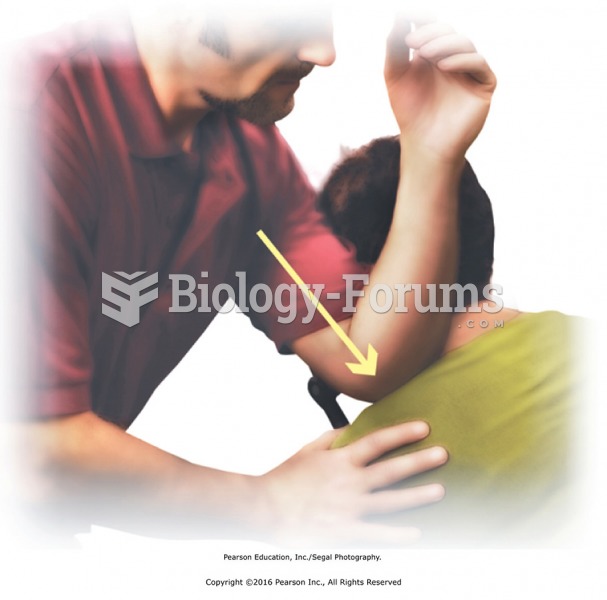 Apply direct pressure to points along the shoulders. Stand to one side and use your forearm to apply ...
Apply direct pressure to points along the shoulders. Stand to one side and use your forearm to apply ...