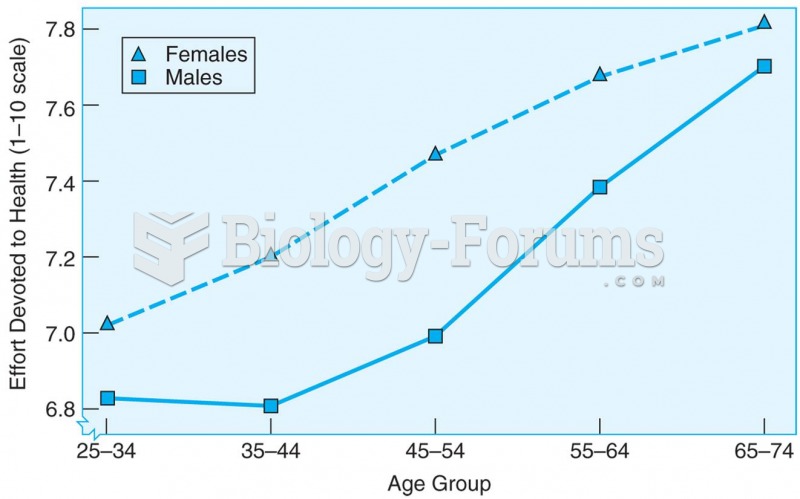
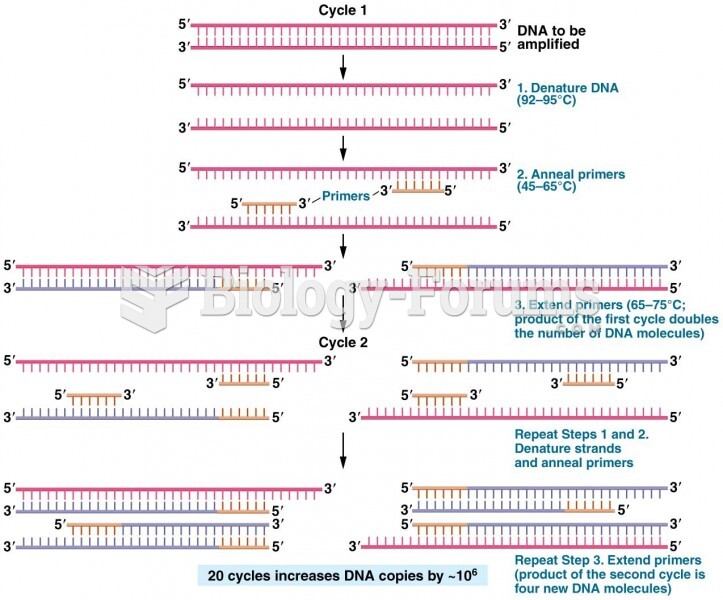
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Vaccines prevent between 2.5 and 4 million deaths every year.
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Did you know?
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
Did you know?
Though “Krazy Glue” or “Super Glue” has the ability to seal small wounds, it is not recommended for this purpose since it contains many substances that should not enter the body through the skin, and may be harmful.
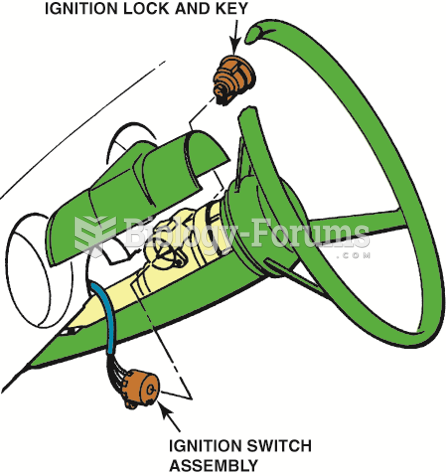 Some column-mounted ignition switches act directly on the contact points, whereas others use a link ...
Some column-mounted ignition switches act directly on the contact points, whereas others use a link ...
 The amount each injector is able to flow is displayed in glass cylinders are each injector for a ...
The amount each injector is able to flow is displayed in glass cylinders are each injector for a ...