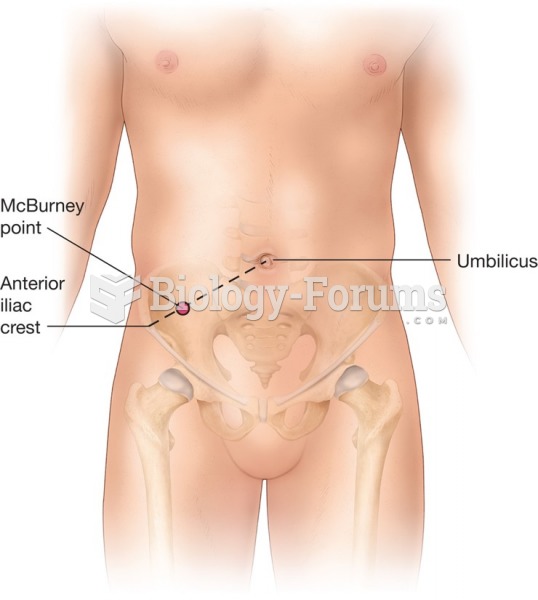
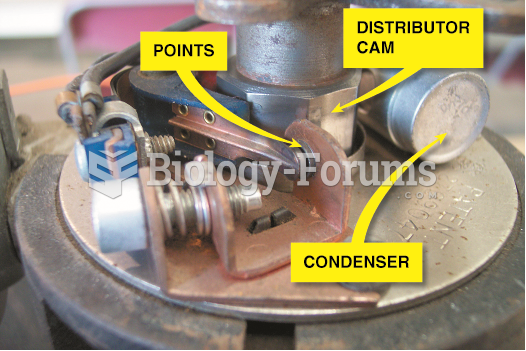
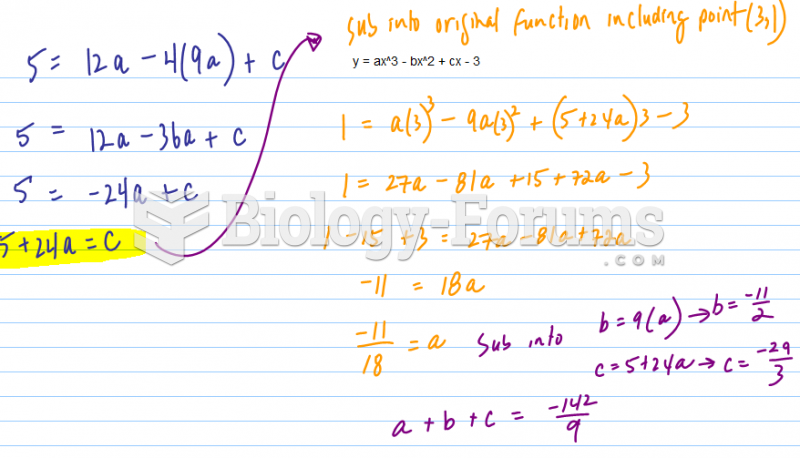
|
|
|
Did you know?
There are more bacteria in your mouth than there are people in the world.
Did you know?
Thyroid conditions cause a higher risk of fibromyalgia and chronic fatigue syndrome.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
Vital signs (blood pressure, temperature, pulse rate, respiration rate) should be taken before any drug administration. Patients should be informed not to use tobacco or caffeine at least 30 minutes before their appointment.
Did you know?
Vampire bats have a natural anticoagulant in their saliva that permits continuous bleeding after they painlessly open a wound with their incisors. This capillary blood does not cause any significant blood loss to their victims.