|
|
|
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
Your heart beats over 36 million times a year.



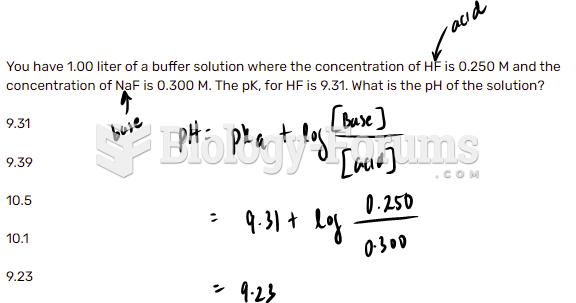
![What is the pH of an aqueous solution if the [H+] = 0.000 000 075 M?](https://biology-forums.com/gallery/43/medium_6_11_12_21_7_23_55.jpeg)