This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.
Did you know?
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.
Did you know?
For about 100 years, scientists thought that peptic ulcers were caused by stress, spicy food, and alcohol. Later, researchers added stomach acid to the list of causes and began treating ulcers with antacids. Now it is known that peptic ulcers are predominantly caused by Helicobacter pylori, a spiral-shaped bacterium that normally exist in the stomach.
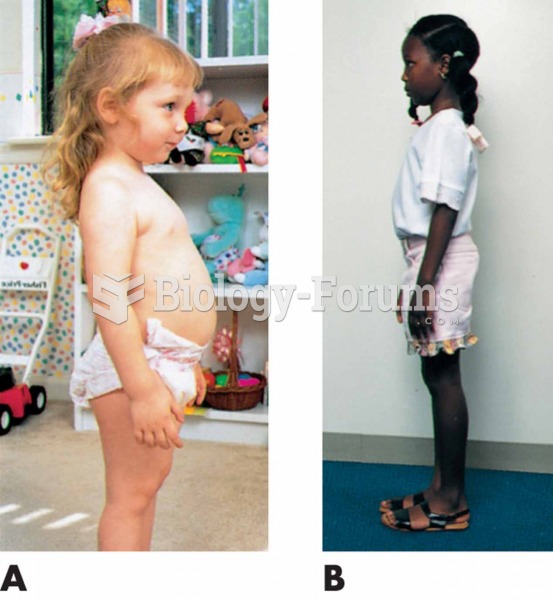 Normal development of posture and spinal curves. (A) Toddler: Protruding abdomen; lumbar lordosis. (
Normal development of posture and spinal curves. (A) Toddler: Protruding abdomen; lumbar lordosis. (
 The Kingsley plantation, on Fort George Island in Jacksonville, Florida. Zephaniah Kingsley, the own
The Kingsley plantation, on Fort George Island in Jacksonville, Florida. Zephaniah Kingsley, the own





