This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Normal urine is sterile. It contains fluids, salts, and waste products. It is free of bacteria, viruses, and fungi.
Did you know?
Computer programs are available that crosscheck a new drug's possible trade name with all other trade names currently available. These programs detect dangerous similarities between names and alert the manufacturer of the drug.
Did you know?
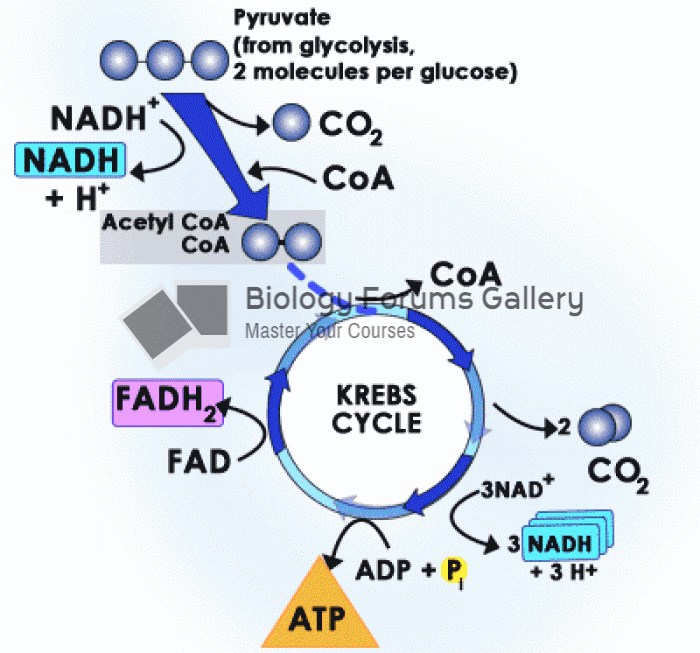
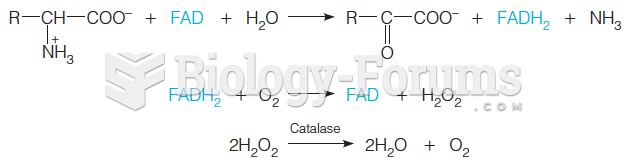
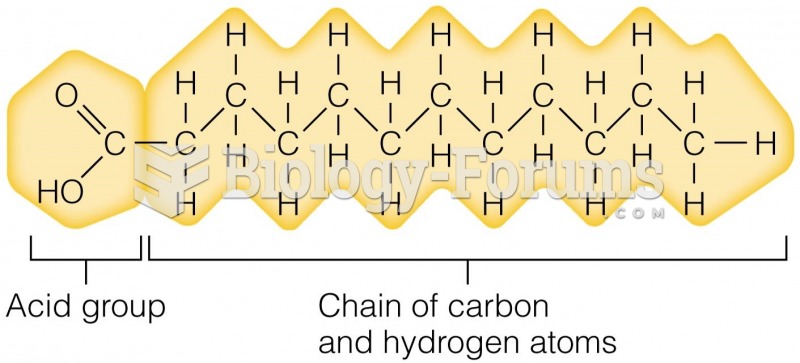
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Did you know?
Everyone has one nostril that is larger than the other.