|
|
|
Astigmatism is the most common vision problem. It may accompany nearsightedness or farsightedness. It is usually caused by an irregularly shaped cornea, but sometimes it is the result of an irregularly shaped lens. Either type can be corrected by eyeglasses, contact lenses, or refractive surgery.
According to the Migraine Research Foundation, migraines are the third most prevalent illness in the world. Women are most affected (18%), followed by children of both sexes (10%), and men (6%).
Illicit drug use costs the United States approximately $181 billion every year.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
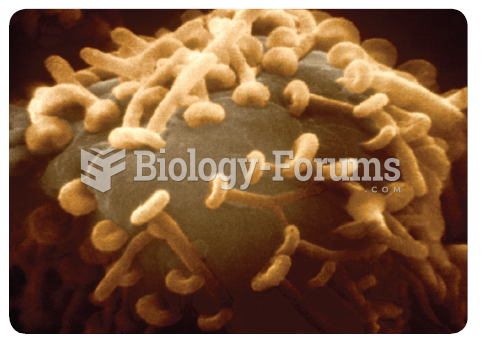
About 80% of major fungal systemic infections are due to Candida albicans. Another form, Candida peritonitis, occurs most often in postoperative patients. A rare disease, Candida meningitis, may follow leukemia, kidney transplant, other immunosuppressed factors, or when suffering from Candida septicemia.
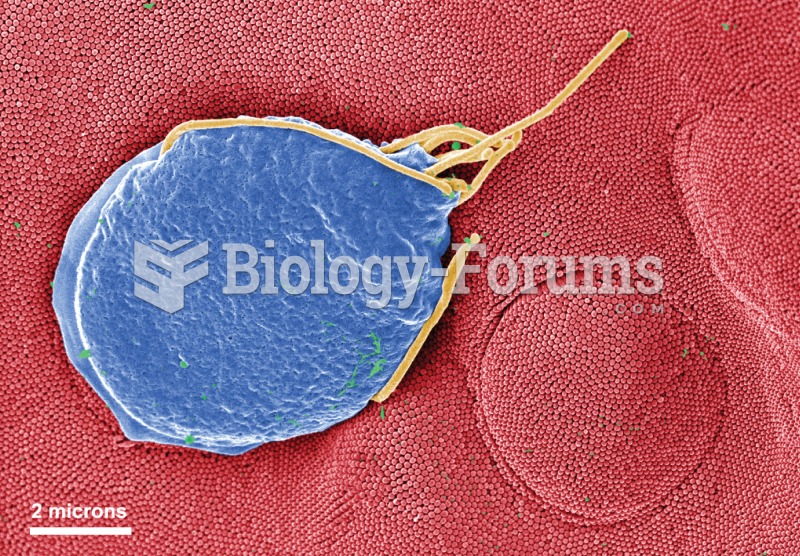 Giardiasis. Colorized electron micrograph of a Giardia protozoan adhering to the surface of an epith
Giardiasis. Colorized electron micrograph of a Giardia protozoan adhering to the surface of an epith
 The paleoanthropologist must understand these deformations in order to figure out which strata a fos
The paleoanthropologist must understand these deformations in order to figure out which strata a fos
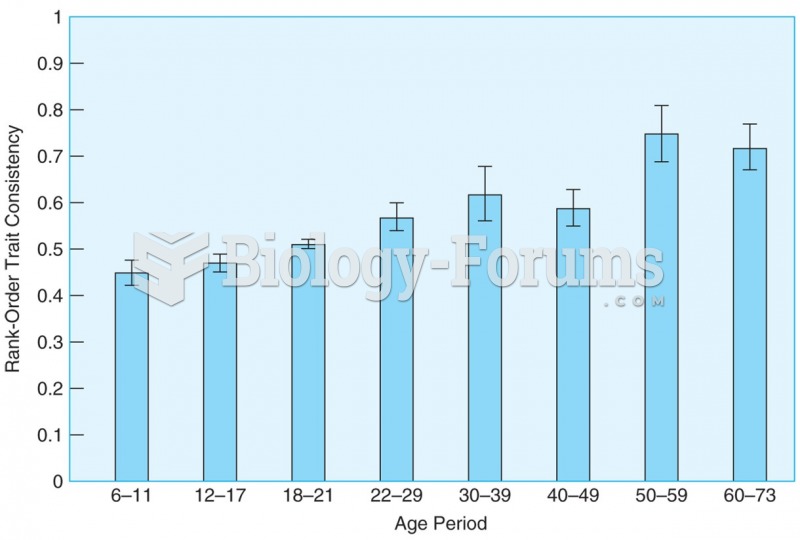 Rank-order correlations show that differential consistency remains high from childhood through late ...
Rank-order correlations show that differential consistency remains high from childhood through late ...