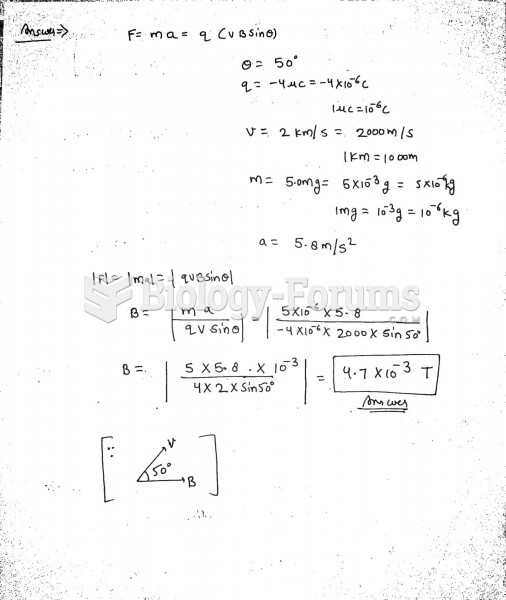
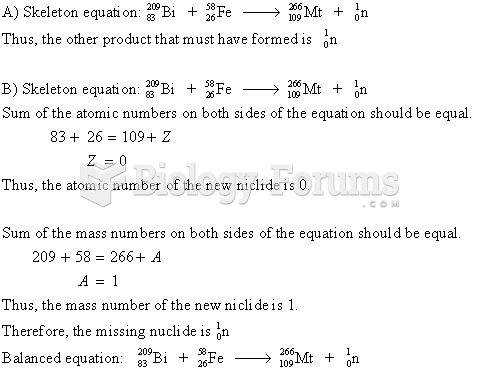
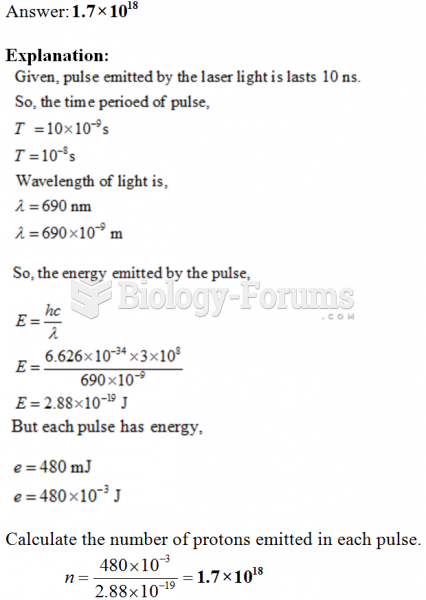This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
Did you know?
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Did you know?
There are more nerve cells in one human brain than there are stars in the Milky Way.