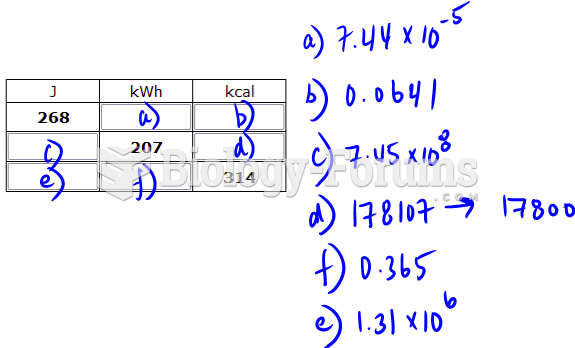
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.
Did you know?
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
Medication errors are three times higher among children and infants than with adults.