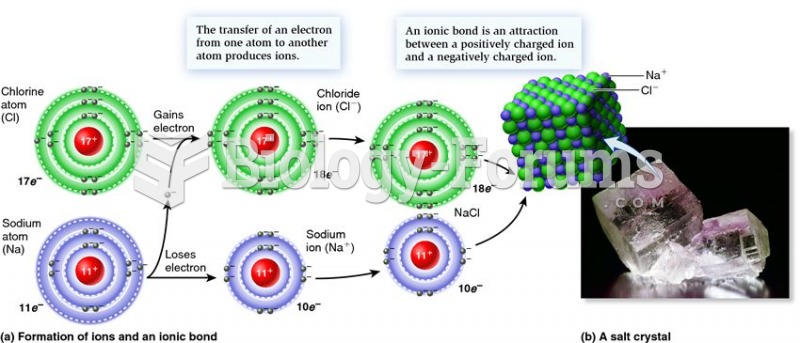
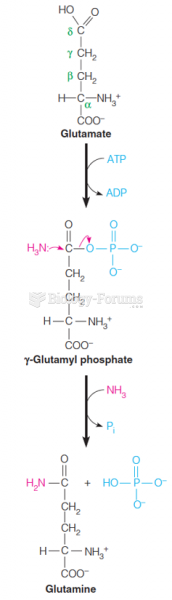
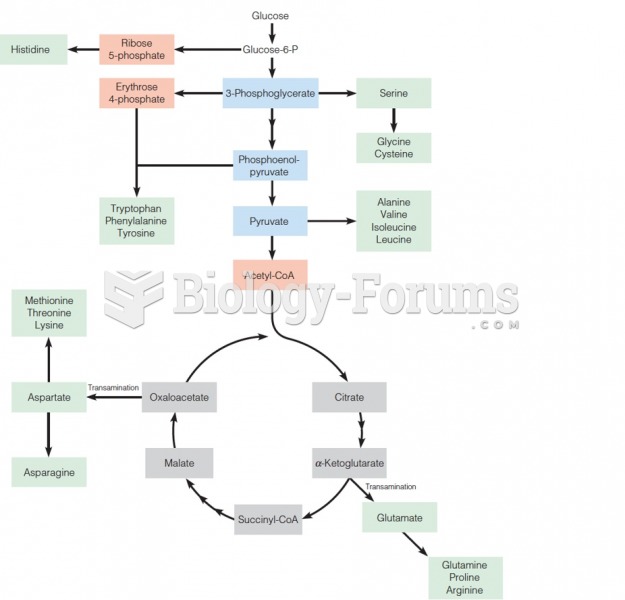
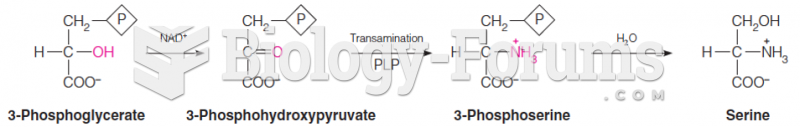
|
|
|
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
To prove that stomach ulcers were caused by bacteria and not by stress, a researcher consumed an entire laboratory beaker full of bacterial culture. After this, he did indeed develop stomach ulcers, and won the Nobel Prize for his discovery.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.