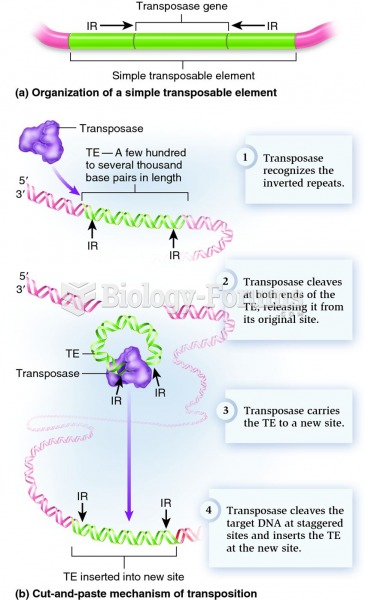
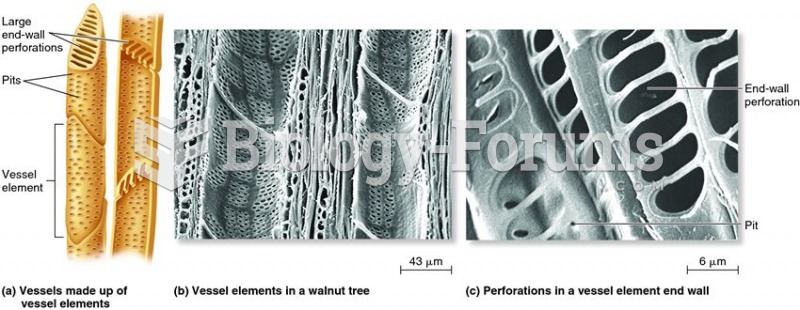
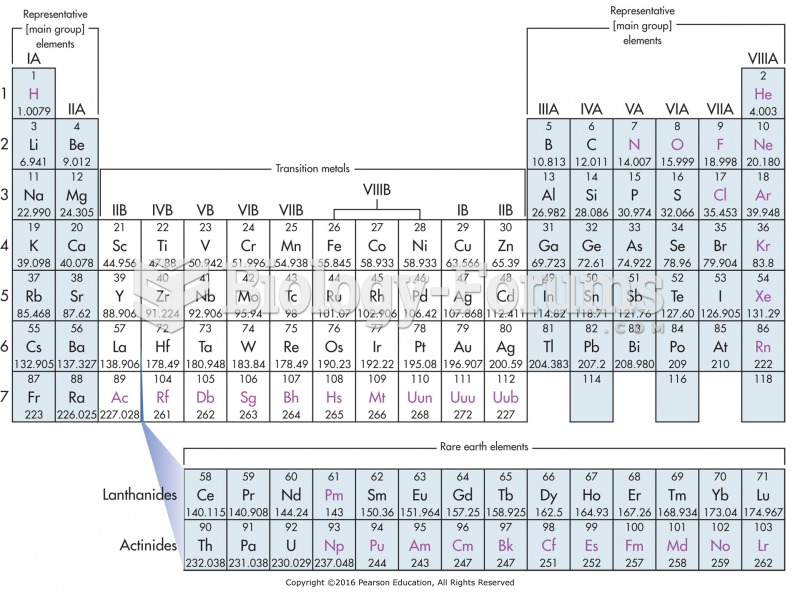
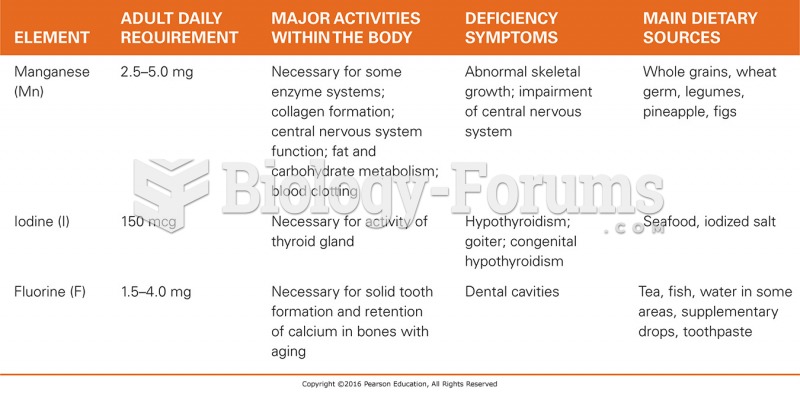
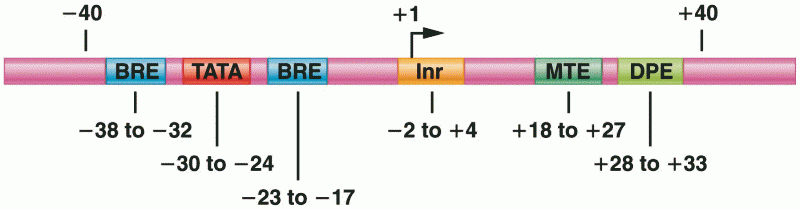
|
|
|
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Medications that are definitely not safe to take when breastfeeding include radioactive drugs, antimetabolites, some cancer (chemotherapy) agents, bromocriptine, ergotamine, methotrexate, and cyclosporine.