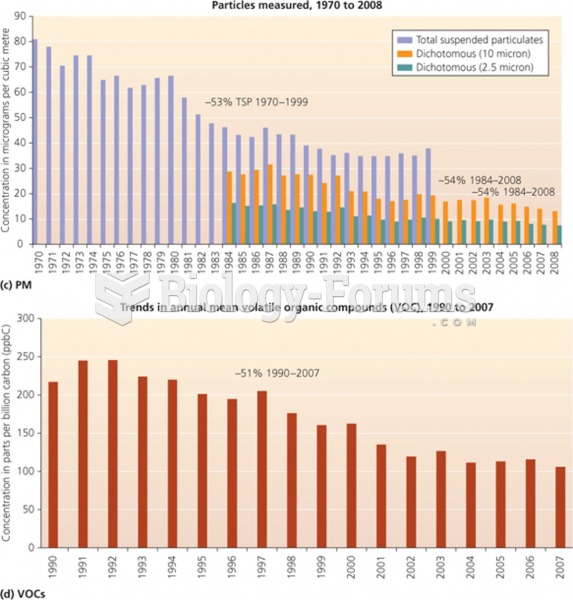
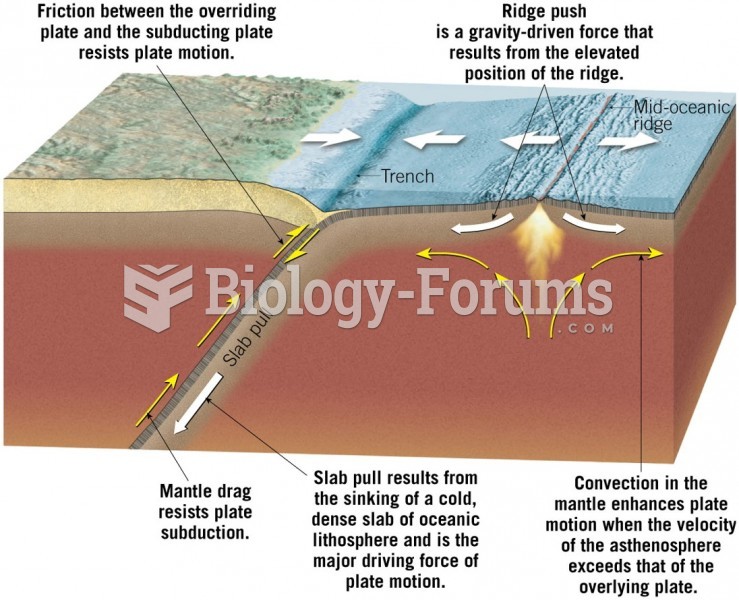
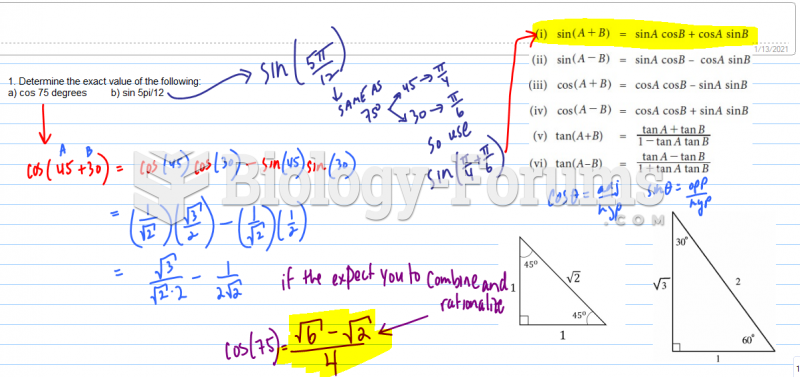
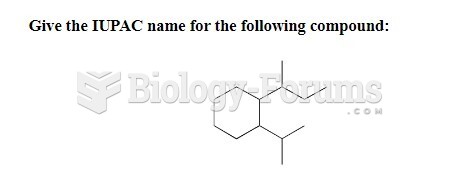
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
In 1844, Charles Goodyear obtained the first patent for a rubber condom.
Did you know?
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
Did you know?
Most women experience menopause in their 50s. However, in 1994, an Italian woman gave birth to a baby boy when she was 61 years old.