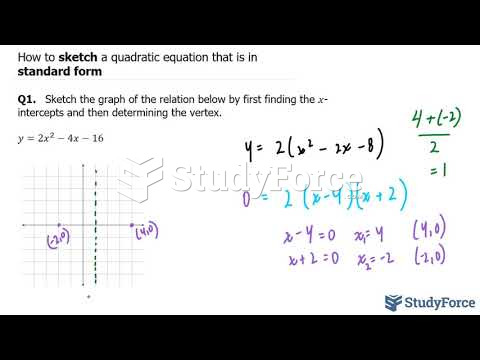
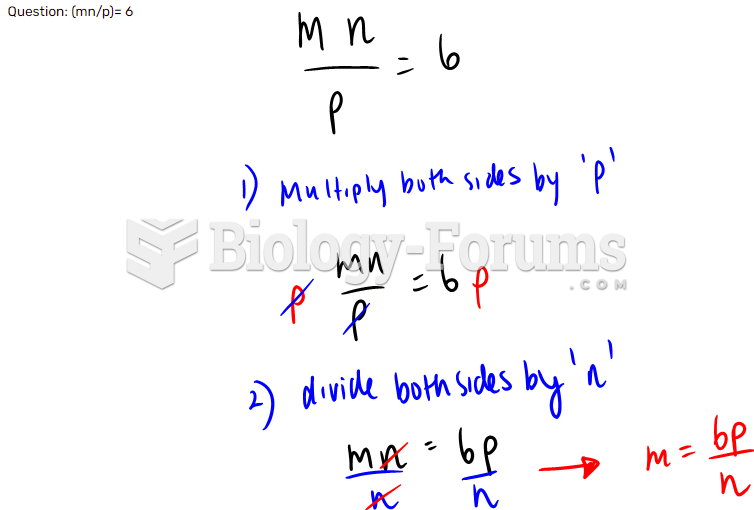
|
|
|
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Sildenafil (Viagra®) has two actions that may be of consequence in patients with heart disease. It can lower the blood pressure, and it can interact with nitrates. It should never be used in patients who are taking nitrates.
Complications of influenza include: bacterial pneumonia, ear and sinus infections, dehydration, and worsening of chronic conditions such as asthma, congestive heart failure, or diabetes.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.