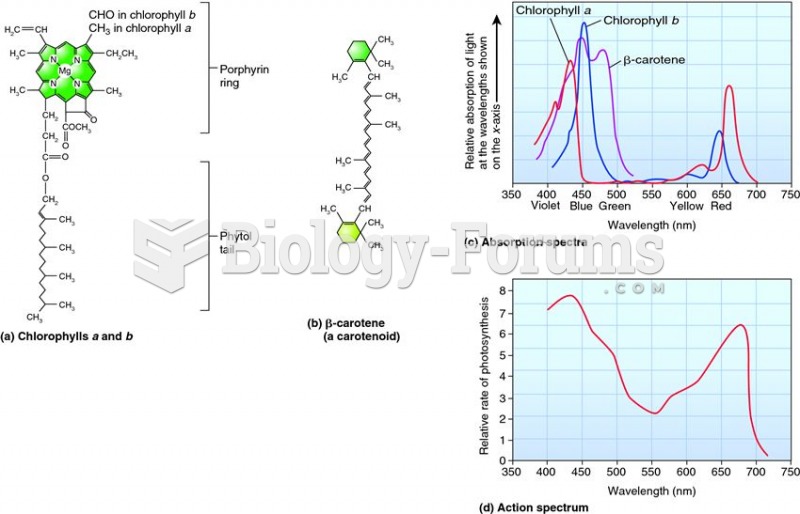
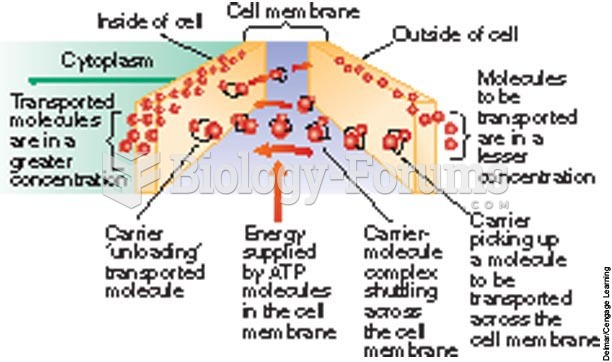
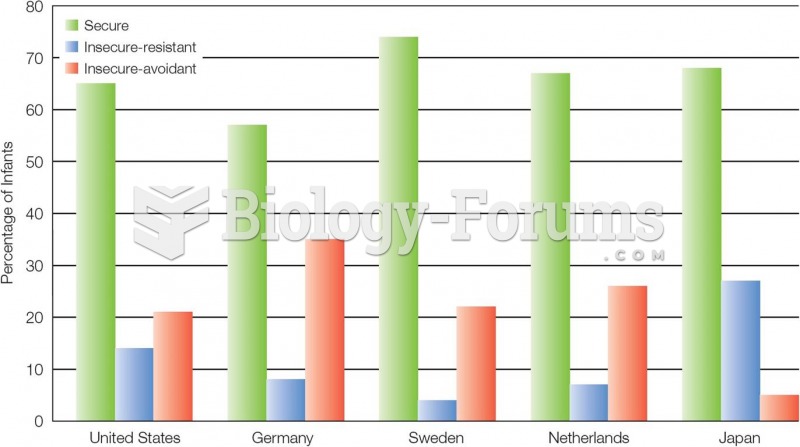
|
|
|
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
The average adult has about 21 square feet of skin.
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.