|
|
|
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Thyroid conditions may make getting pregnant impossible.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
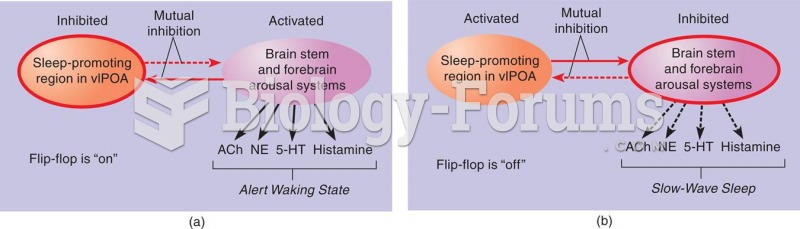
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.