|
|
|
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
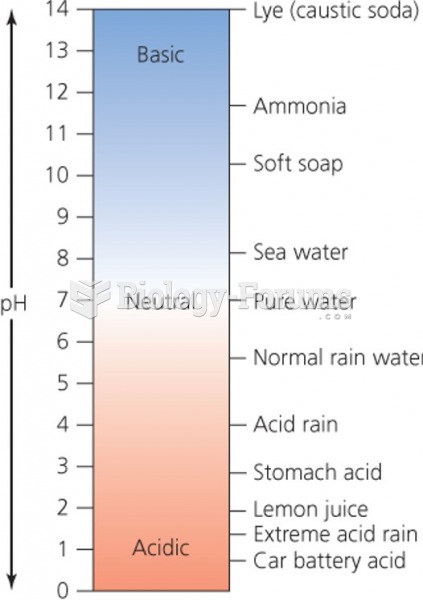
On average, the stomach produces 2 L of hydrochloric acid per day.
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
The Food and Drug Administration has approved Risperdal, an adult antipsychotic drug, for the symptomatic treatment of irritability in children and adolescents with autism. The approval is the first for the use of a drug to treat behaviors associated with autism in children. These behaviors are included under the general heading of irritability and include aggression, deliberate self-injury, and temper tantrums.