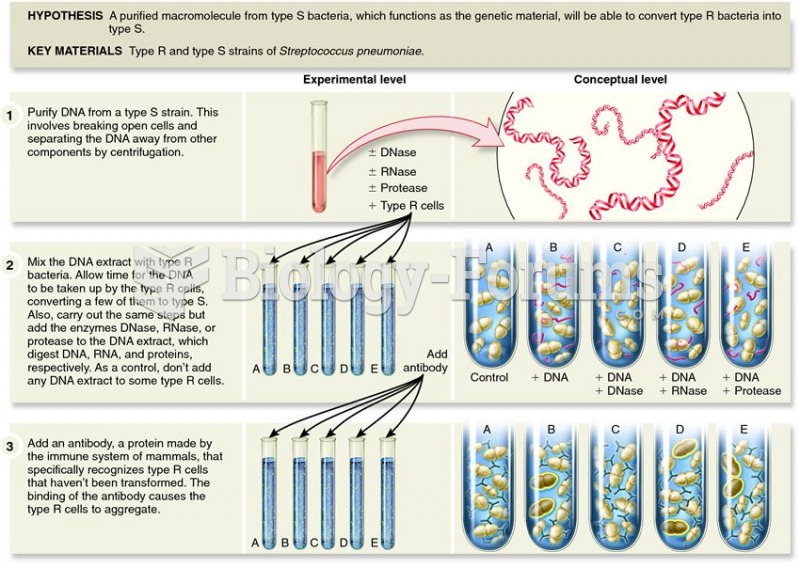
|
|
|
The cure for trichomoniasis is easy as long as the patient does not drink alcoholic beverages for 24 hours. Just a single dose of medication is needed to rid the body of the disease. However, without proper precautions, an individual may contract the disease repeatedly. In fact, most people develop trichomoniasis again within three months of their last treatment.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Acetaminophen (Tylenol) in overdose can seriously damage the liver. It should never be taken by people who use alcohol heavily; it can result in severe liver damage and even a condition requiring a liver transplant.
In 1864, the first barbiturate (barbituric acid) was synthesized.