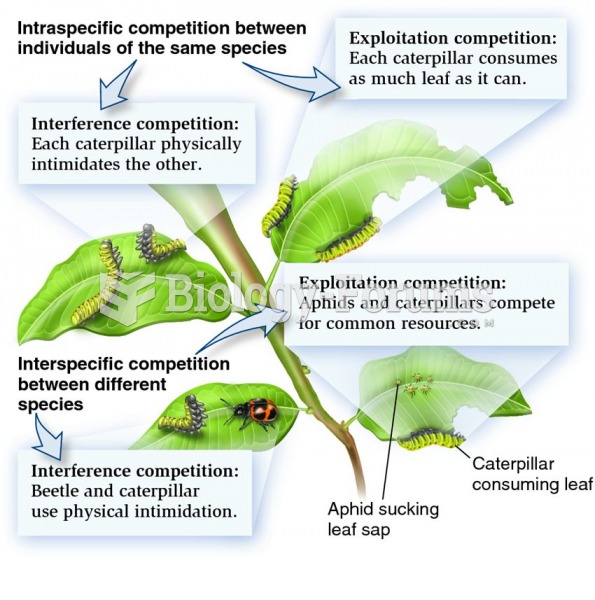
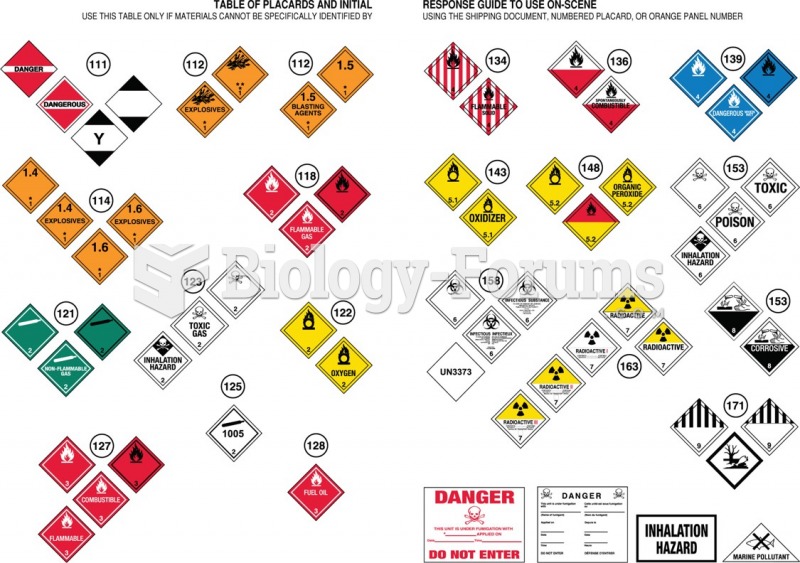
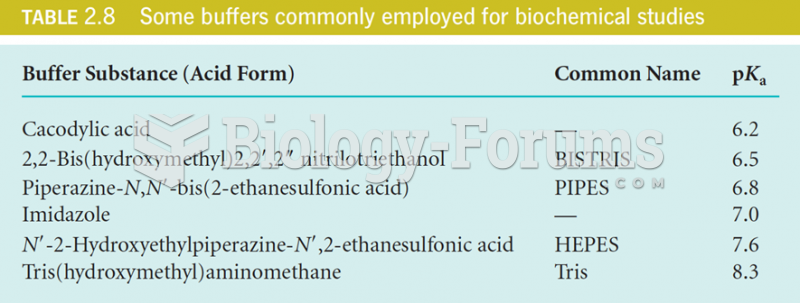
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
Your heart beats over 36 million times a year.
Did you know?
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.