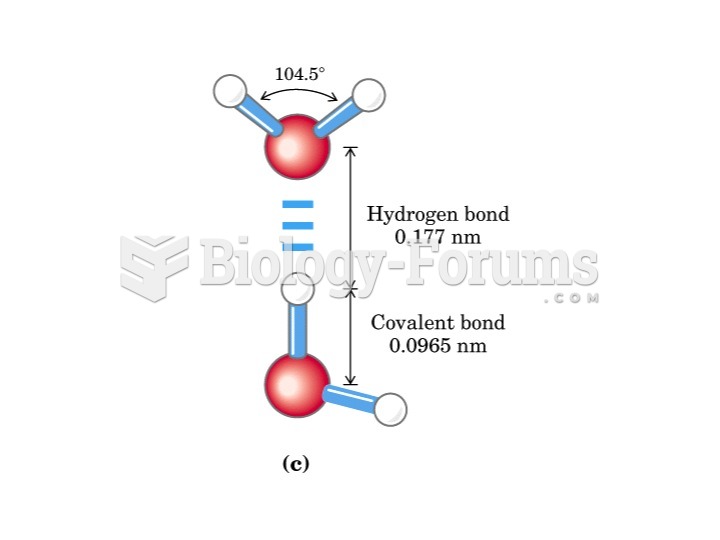
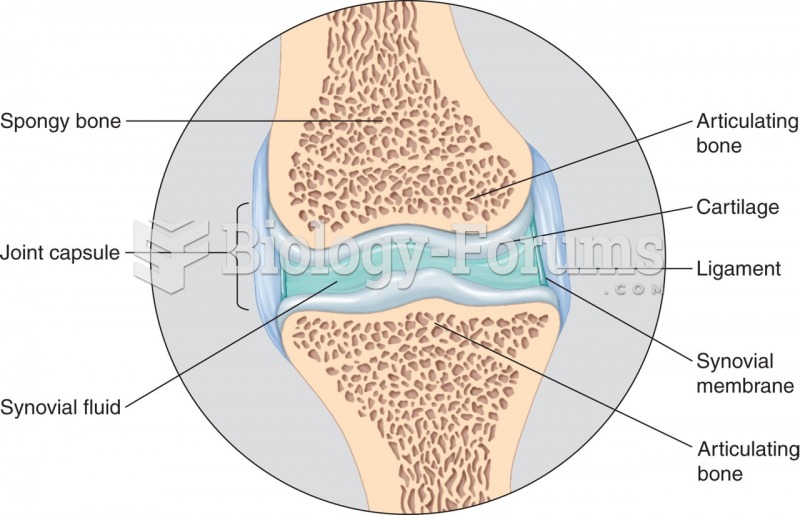
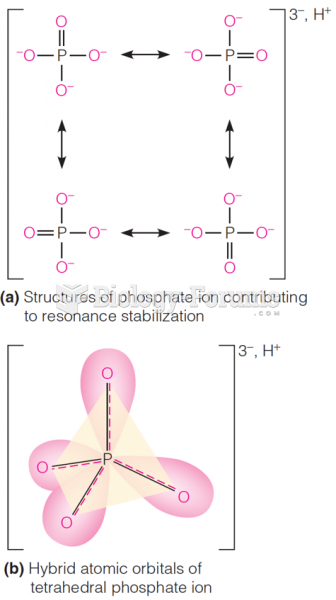
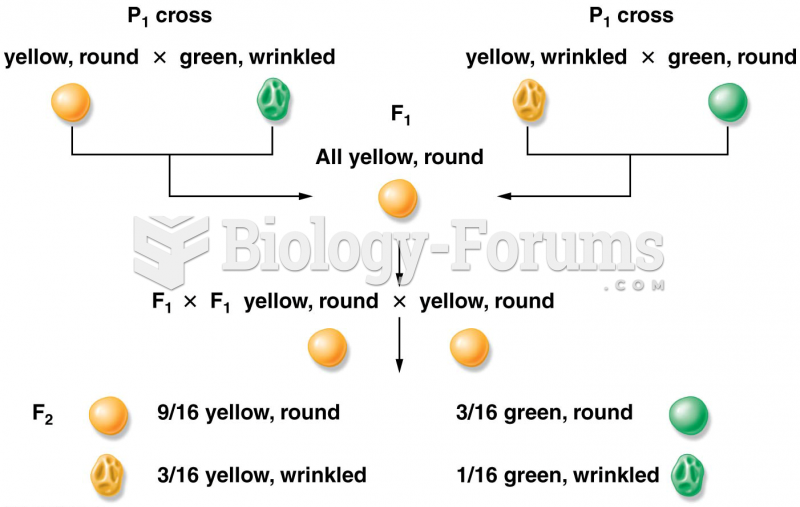
|
|
|
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
In inpatient settings, adverse drug events account for an estimated one in three of all hospital adverse events. They affect approximately 2 million hospital stays every year, and prolong hospital stays by between one and five days.
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Never take aspirin without food because it is likely to irritate your stomach. Never give aspirin to children under age 12. Overdoses of aspirin have the potential to cause deafness.