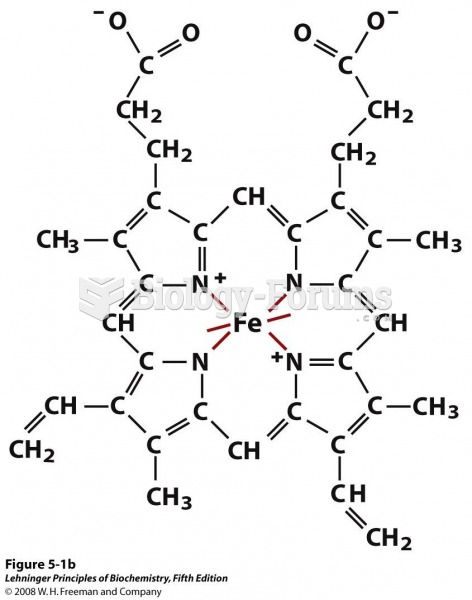
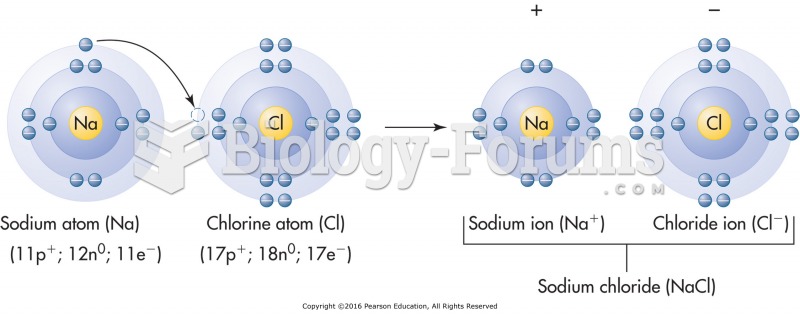
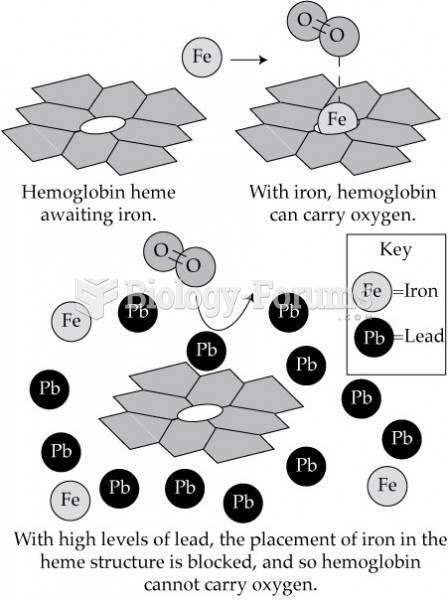
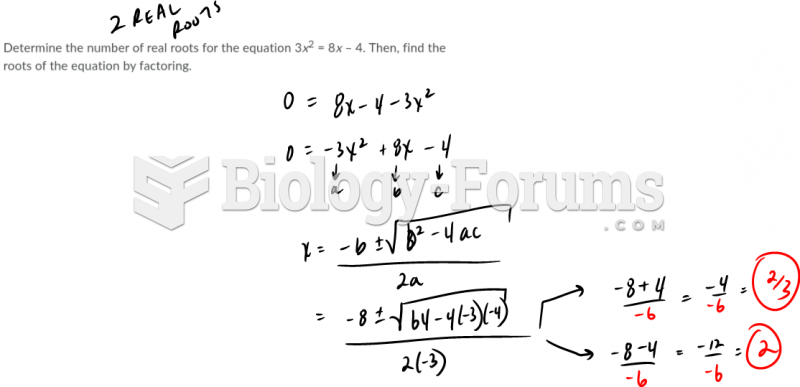
|
|
|
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Most strokes are caused when blood clots move to a blood vessel in the brain and block blood flow to that area. Thrombolytic therapy can be used to dissolve the clot quickly. If given within 3 hours of the first stroke symptoms, this therapy can help limit stroke damage and disability.
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Long-term mental and physical effects from substance abuse include: paranoia, psychosis, immune deficiencies, and organ damage.