This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
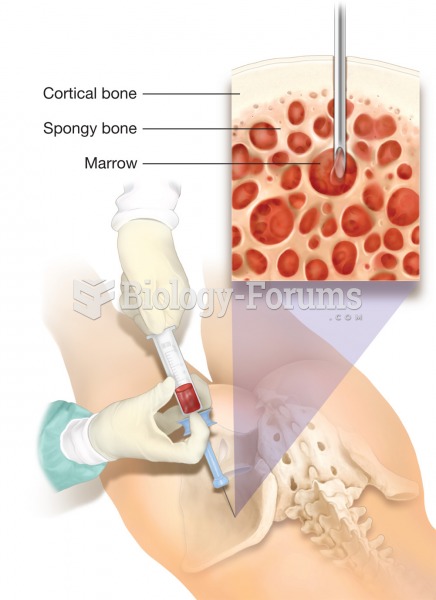
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Did you know?
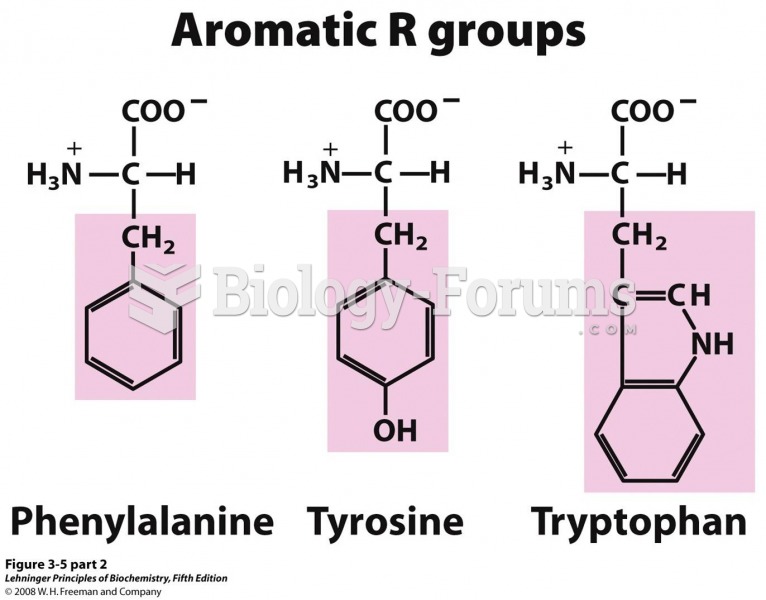
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
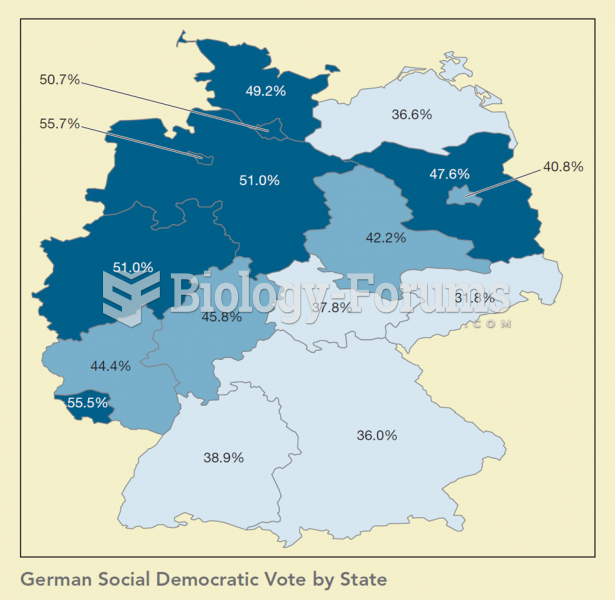
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
Did you know?
Increased intake of vitamin D has been shown to reduce fractures up to 25% in older people.