|
|
|
Your chance of developing a kidney stone is 1 in 10. In recent years, approximately 3.7 million people in the United States were diagnosed with a kidney disease.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
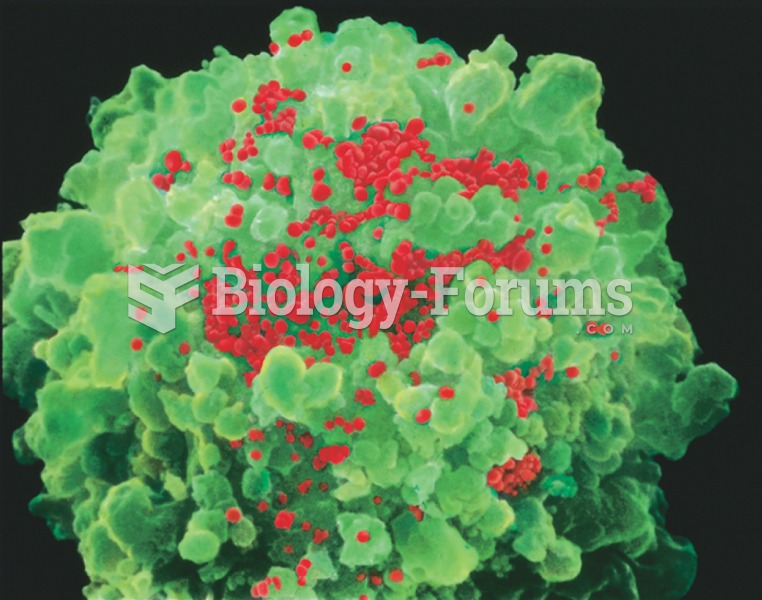
Cancer has been around as long as humankind, but only in the second half of the twentieth century did the number of cancer cases explode.
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
About 3.2 billion people, nearly half the world population, are at risk for malaria. In 2015, there are about 214 million malaria cases and an estimated 438,000 malaria deaths.