|
|
|
When Gabriel Fahrenheit invented the first mercury thermometer, he called "zero degrees" the lowest temperature he was able to attain with a mixture of ice and salt. For the upper point of his scale, he used 96°, which he measured as normal human body temperature (we know it to be 98.6° today because of more accurate thermometers).
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.
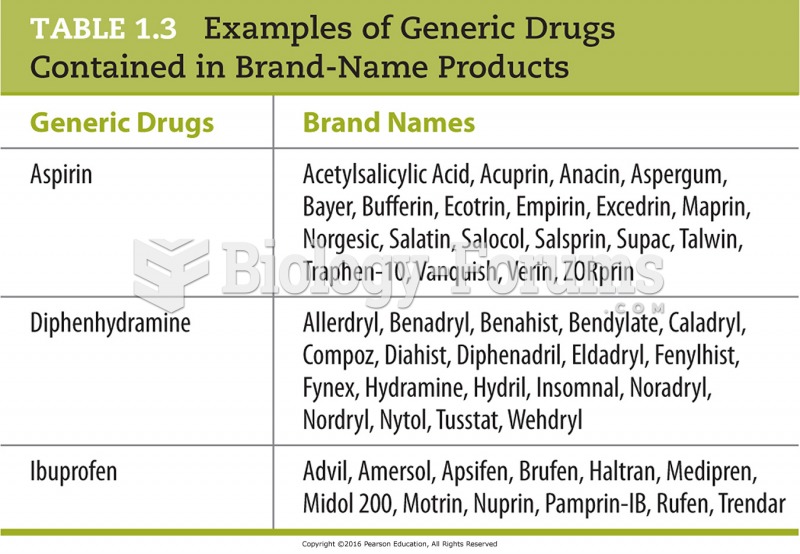
In 2006, a generic antinausea drug named ondansetron was approved. It is used to stop nausea and vomiting associated with surgery, chemotherapy, and radiation therapy.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.