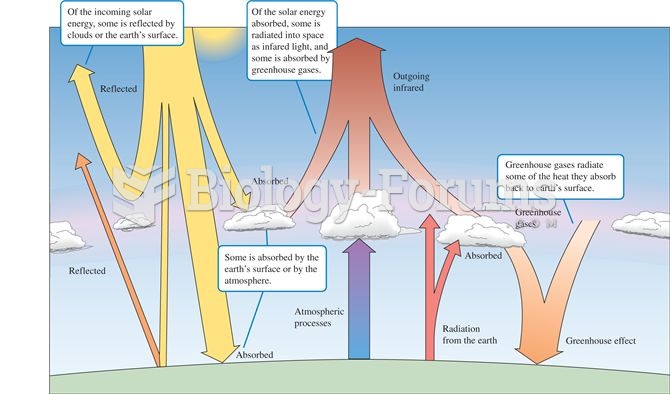
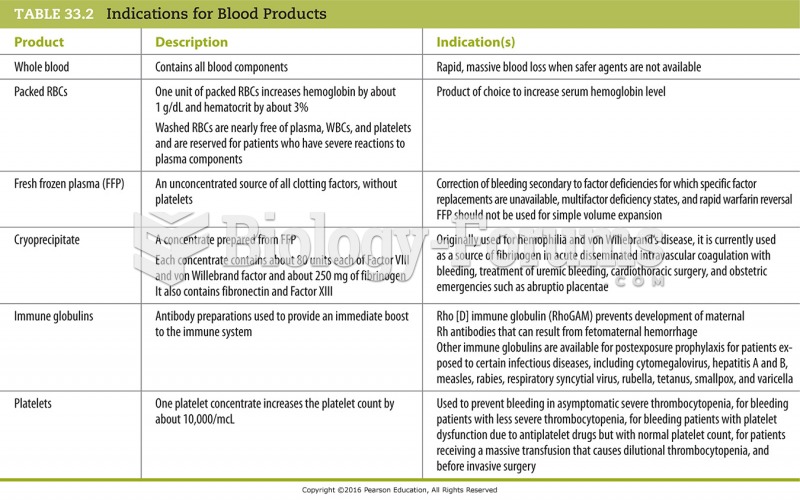
|
|
|
Did you know?
Nitroglycerin is used to alleviate various heart-related conditions, and it is also the chief component of dynamite (but mixed in a solid clay base to stabilize it).
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Did you know?
Cytomegalovirus affects nearly the same amount of newborns every year as Down syndrome.