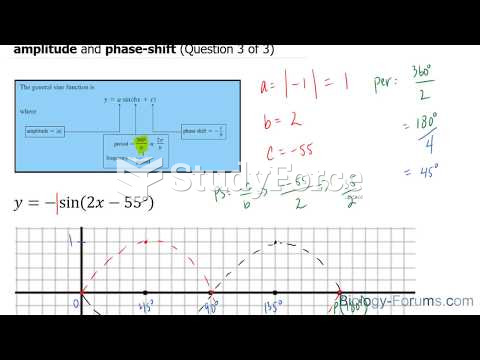
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
 Max Weber (1864–1920) was another early sociologist who left a profound impression on sociology. ...
Max Weber (1864–1920) was another early sociologist who left a profound impression on sociology. ...
 According to Kohlberg’s theory, only a few individuals, such as Martin Luther King, Jr. (left) and ...
According to Kohlberg’s theory, only a few individuals, such as Martin Luther King, Jr. (left) and ...
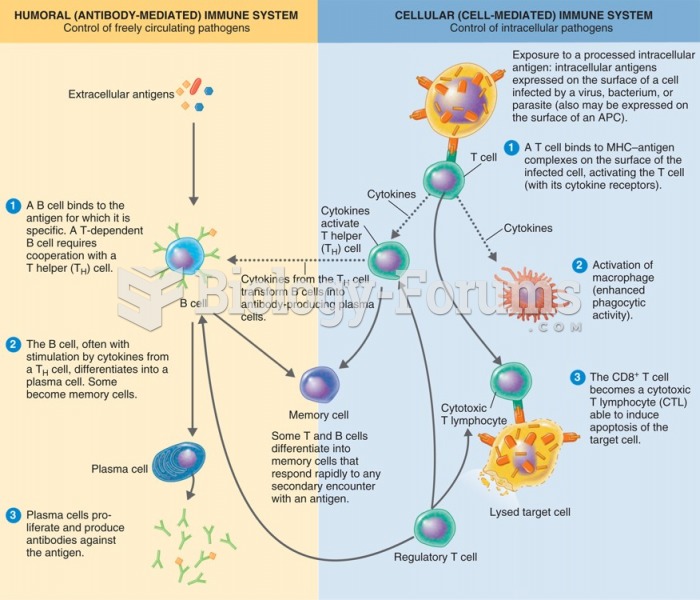 The humoral and cellular immune response. At the left is the humoral (or B-cell mediated); at the ...
The humoral and cellular immune response. At the left is the humoral (or B-cell mediated); at the ...