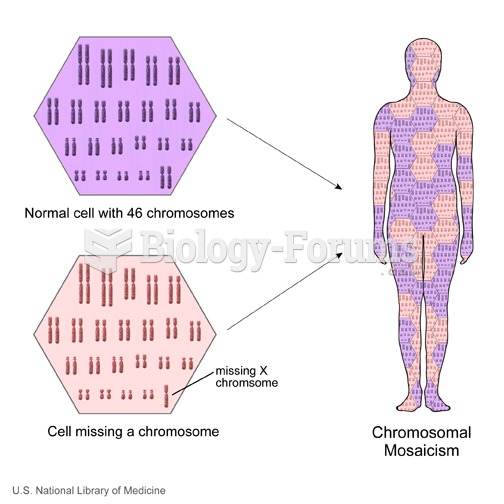
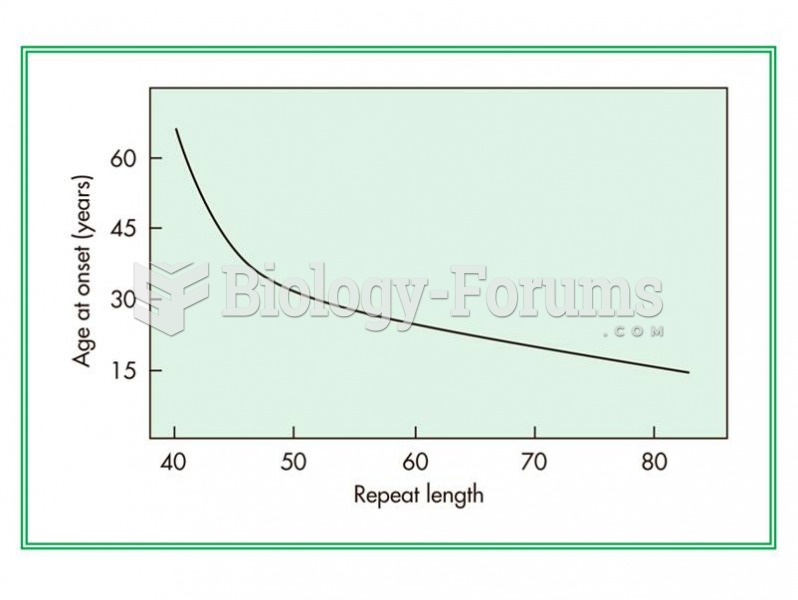
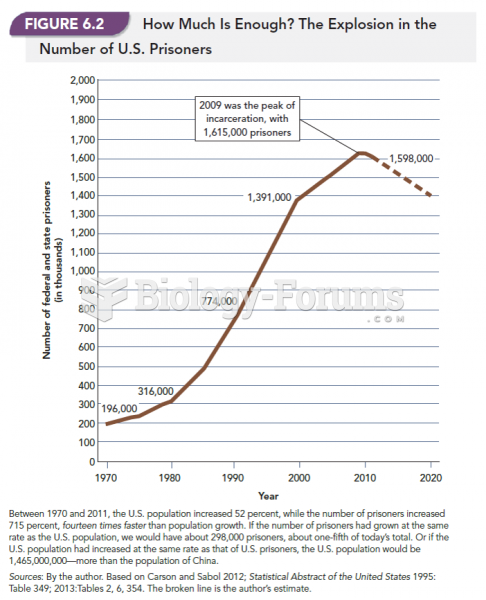
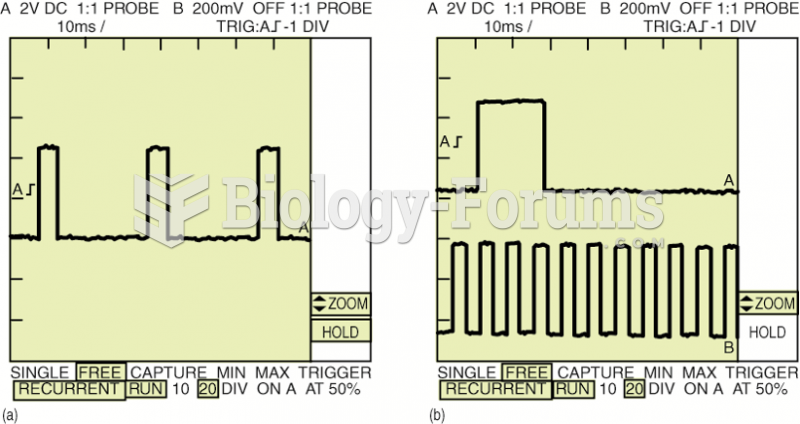
|
|
|
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
People who have myopia, or nearsightedness, are not able to see objects at a distance but only up close. It occurs when the cornea is either curved too steeply, the eye is too long, or both. This condition is progressive and worsens with time. More than 100 million people in the United States are nearsighted, but only 20% of those are born with the condition. Diet, eye exercise, drug therapy, and corrective lenses can all help manage nearsightedness.
When blood is deoxygenated and flowing back to the heart through the veins, it is dark reddish-blue in color. Blood in the arteries that is oxygenated and flowing out to the body is bright red. Whereas arterial blood comes out in spurts, venous blood flows.
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
There are more sensory neurons in the tongue than in any other part of the body.