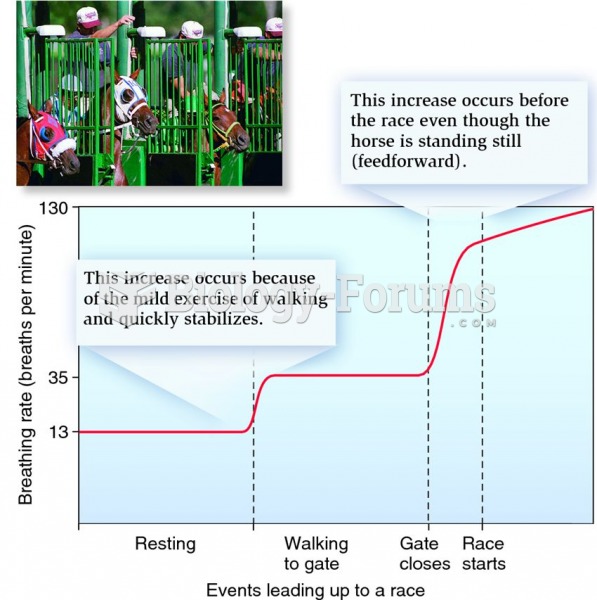
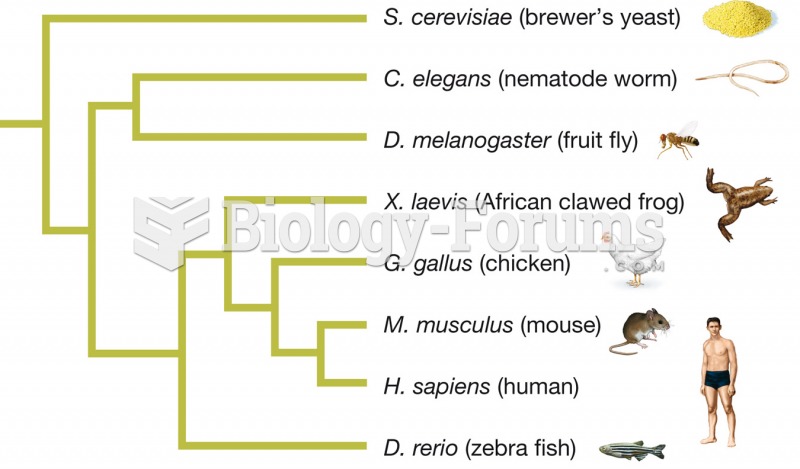
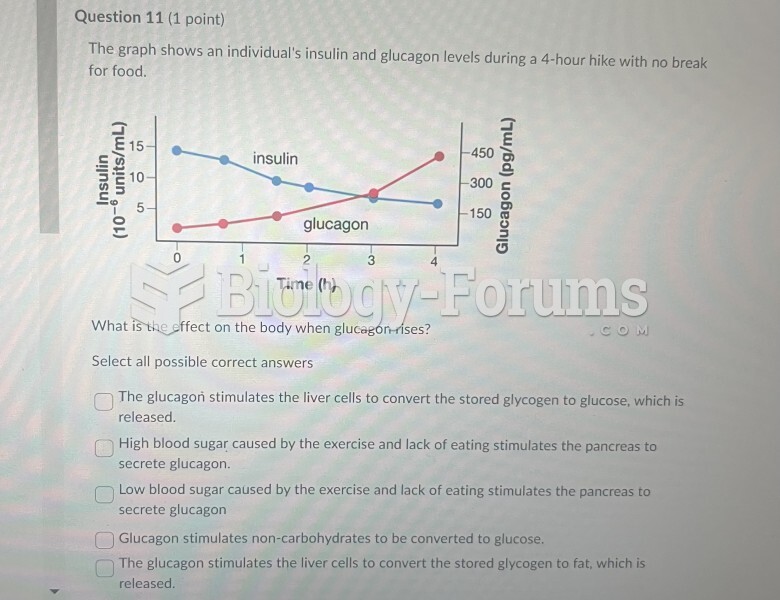
|
|
|
Did you know?
The types of cancer that alpha interferons are used to treat include hairy cell leukemia, melanoma, follicular non-Hodgkin's lymphoma, and AIDS-related Kaposi's sarcoma.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.
Did you know?
Urine turns bright yellow if larger than normal amounts of certain substances are consumed; one of these substances is asparagus.
Did you know?
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.