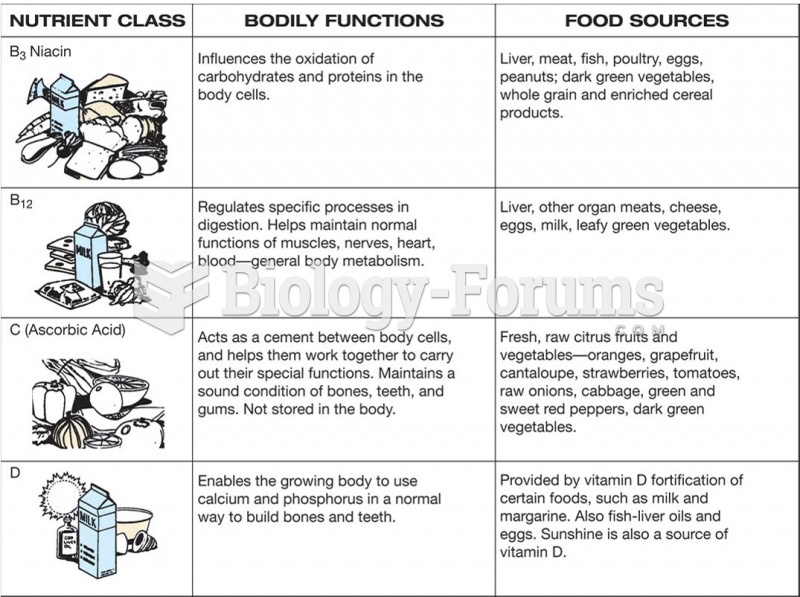
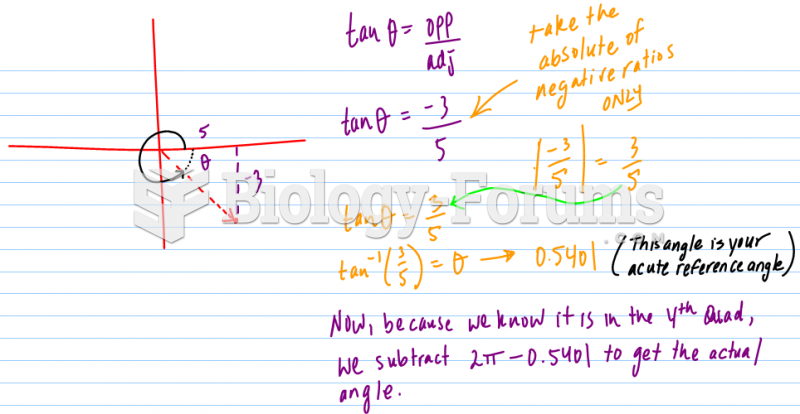
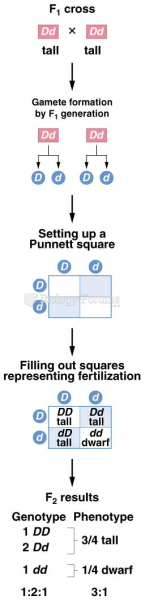
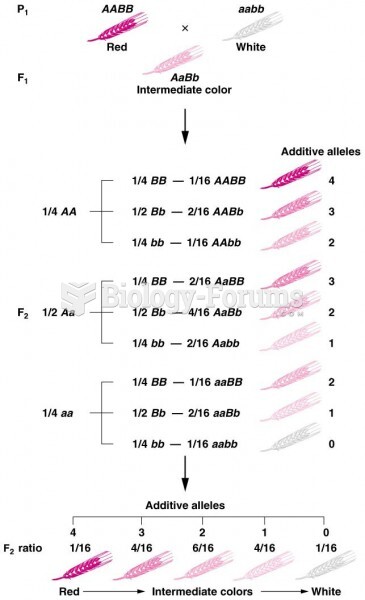
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
More than 4.4billion prescriptions were dispensed within the United States in 2016.
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The average office desk has 400 times more bacteria on it than a toilet.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.