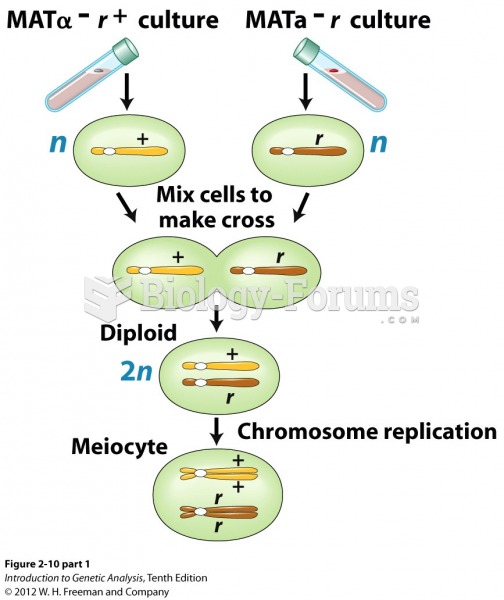
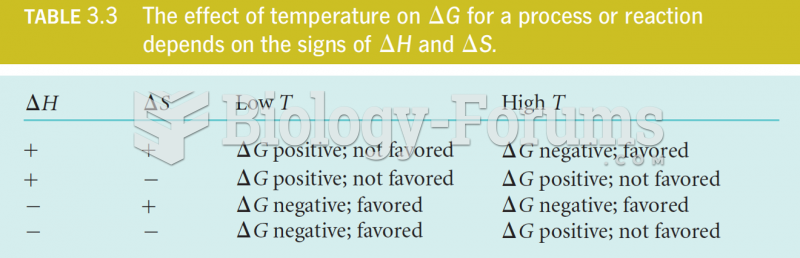
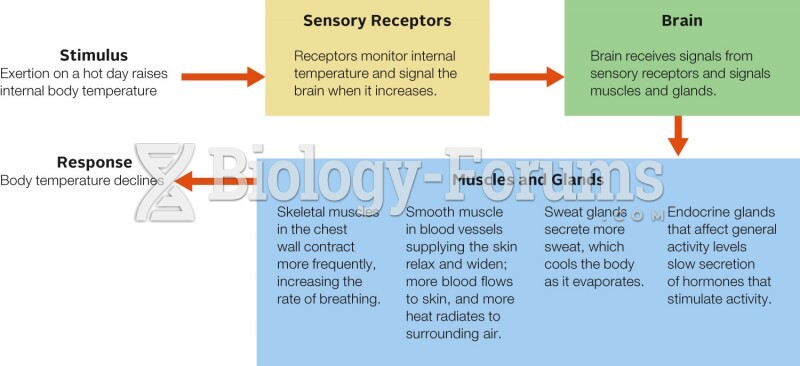
|
|
|
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.
Hypertension is a silent killer because it is deadly and has no significant early symptoms. The danger from hypertension is the extra load on the heart, which can lead to hypertensive heart disease and kidney damage. This occurs without any major symptoms until the high blood pressure becomes extreme. Regular blood pressure checks are an important method of catching hypertension before it can kill you.
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.