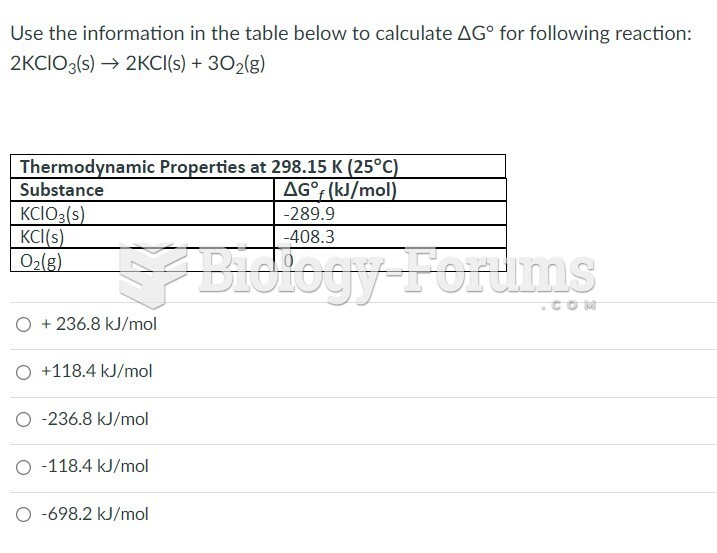
|
|
|
Adolescents often feel clumsy during puberty because during this time of development, their hands and feet grow faster than their arms and legs do. The body is therefore out of proportion. One out of five adolescents actually experiences growing pains during this period.
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Pregnant women usually experience a heightened sense of smell beginning late in the first trimester. Some experts call this the body's way of protecting a pregnant woman from foods that are unsafe for the fetus.
Hip fractures are the most serious consequences of osteoporosis. The incidence of hip fractures increases with each decade among patients in their 60s to patients in their 90s for both women and men of all populations. Men and women older than 80 years of age show the highest incidence of hip fractures.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.