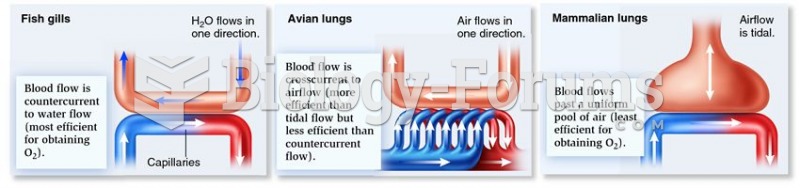
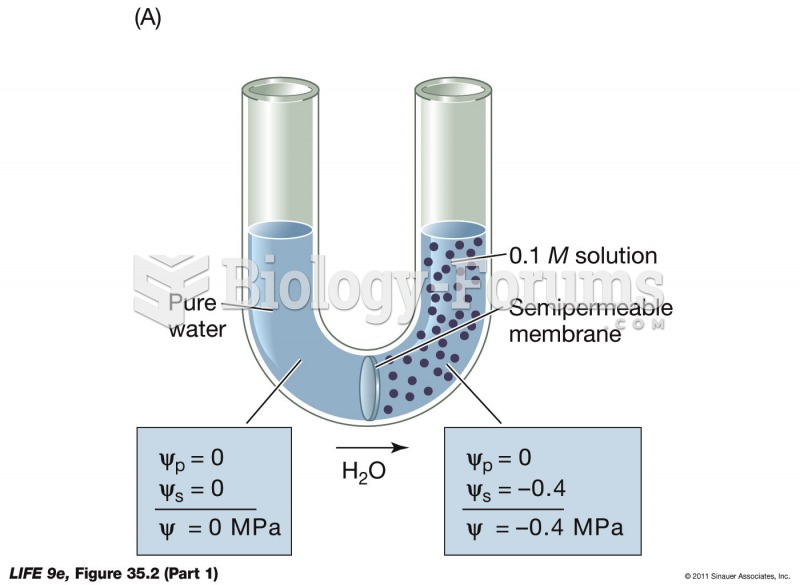
|
|
|
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.