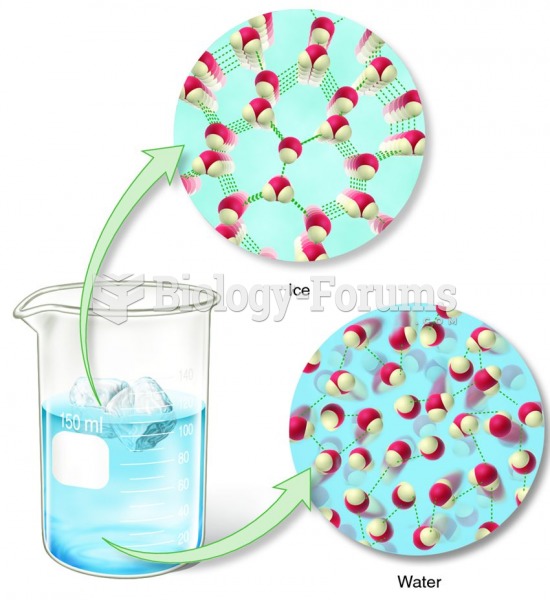
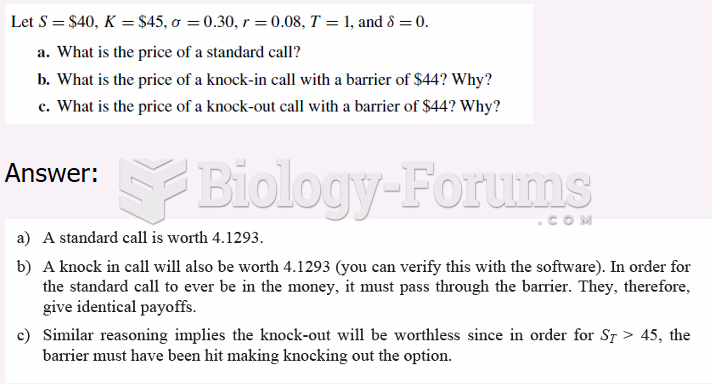
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
Between 1999 and 2012, American adults with high total cholesterol decreased from 18.3% to 12.9%
Did you know?
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
Did you know?
In women, pharmacodynamic differences include increased sensitivity to (and increased effectiveness of) beta-blockers, opioids, selective serotonin reuptake inhibitors, and typical antipsychotics.