This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most common childhood diseases include croup, chickenpox, ear infections, flu, pneumonia, ringworm, respiratory syncytial virus, scabies, head lice, and asthma.
Did you know?
Everyone has one nostril that is larger than the other.
Did you know?
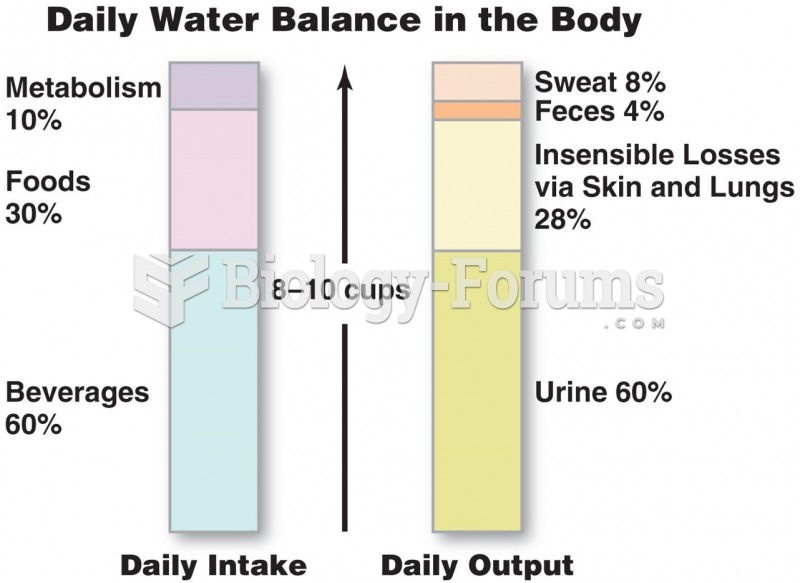
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.
Did you know?
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.