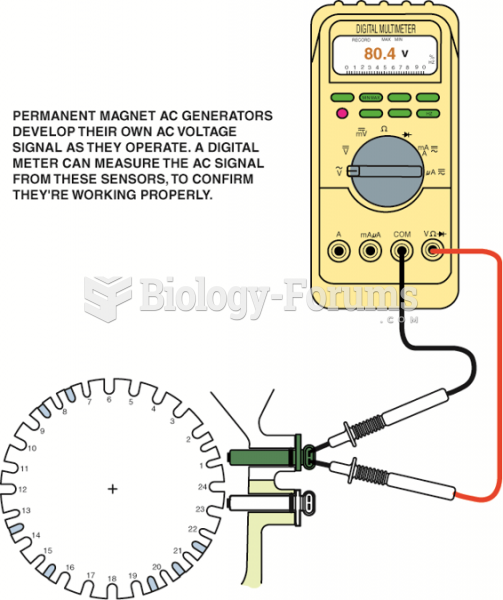
|
|
|
Did you know?
According to the CDC, approximately 31.7% of the U.S. population has high low-density lipoprotein (LDL) or "bad cholesterol" levels.
Did you know?
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
The familiar sounds of your heart are made by the heart's valves as they open and close.
Did you know?
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
 Women have the role of kinkeeper in almost all cultures. This may cause them to react to stress by ...
Women have the role of kinkeeper in almost all cultures. This may cause them to react to stress by ...
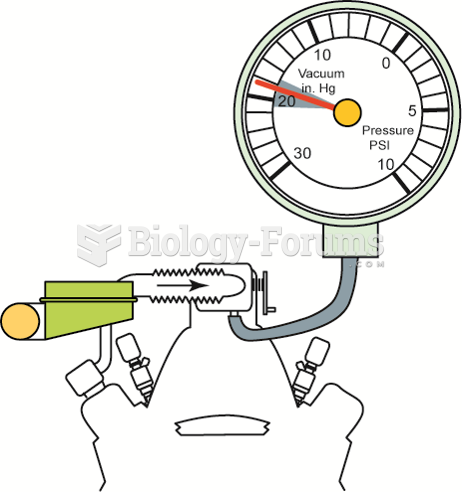 An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...
An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...