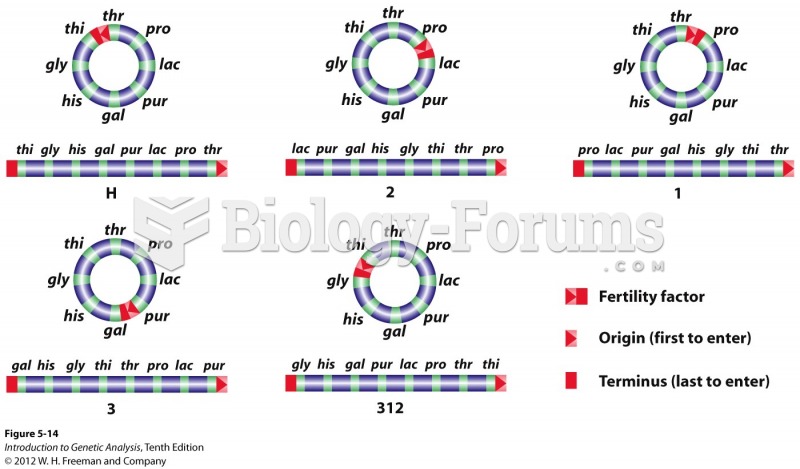
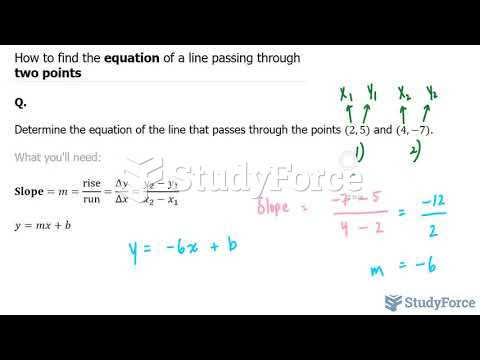
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Did you know?
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
The B-complex vitamins and vitamin C are not stored in the body and must be replaced each day.