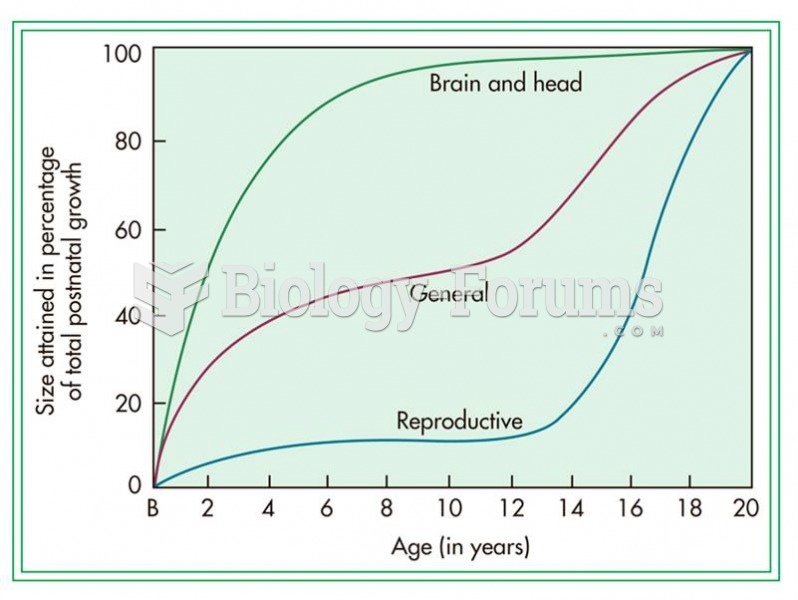
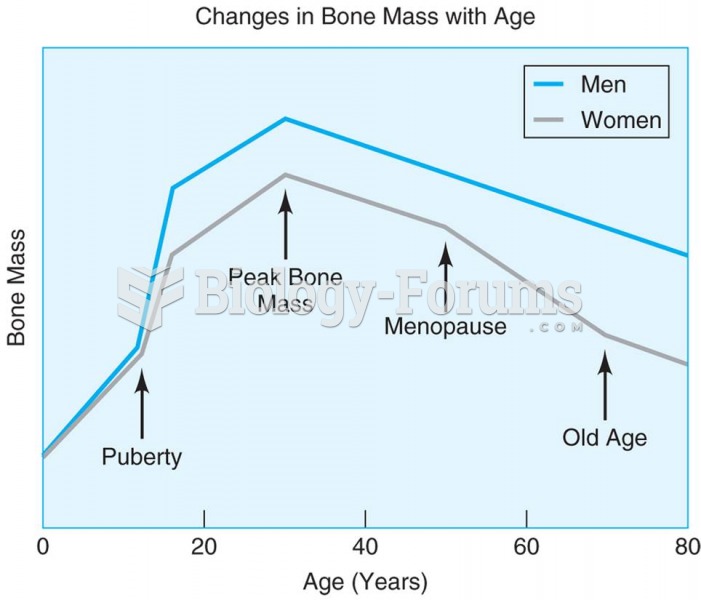
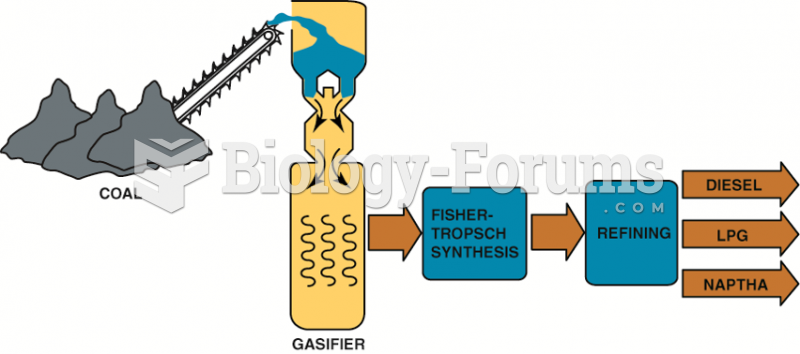
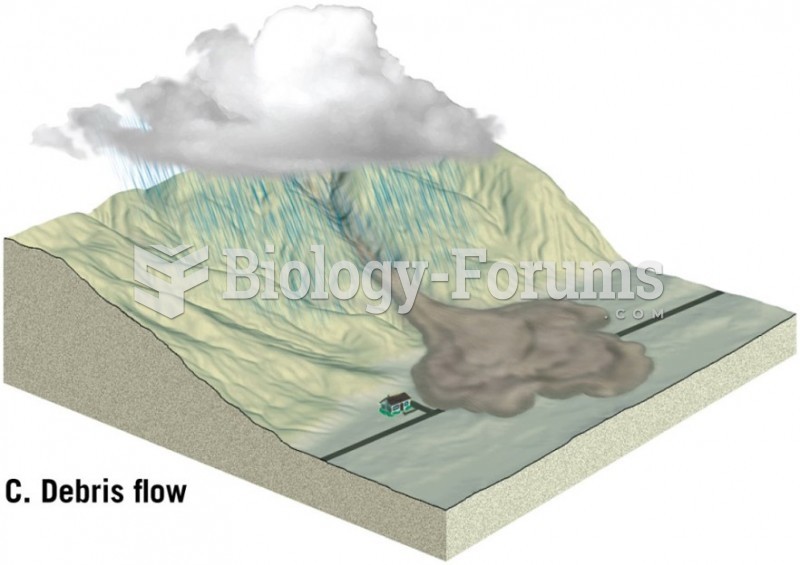
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.
Did you know?
If you could remove all of your skin, it would weigh up to 5 pounds.
Did you know?
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Did you know?
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Did you know?
Human kidneys will clean about 1 million gallons of blood in an average lifetime.