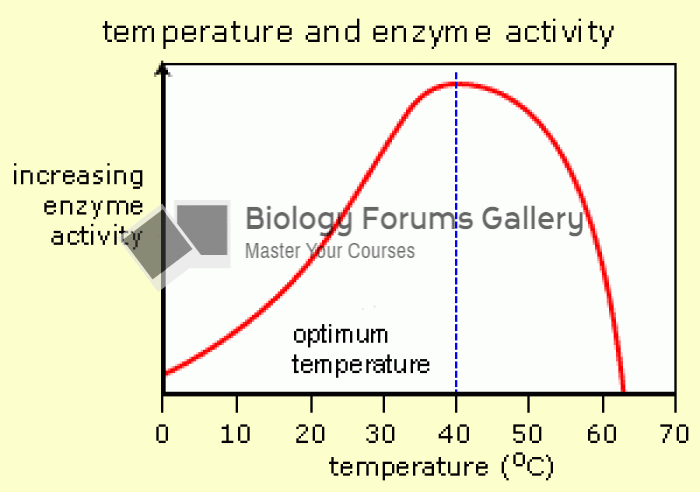
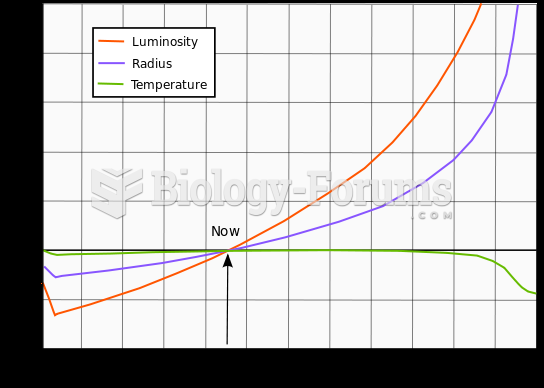
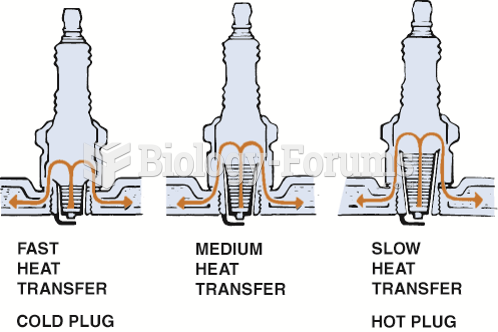
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
The calories found in one piece of cherry cheesecake could light a 60-watt light bulb for 1.5 hours.
Did you know?
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Did you know?
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.