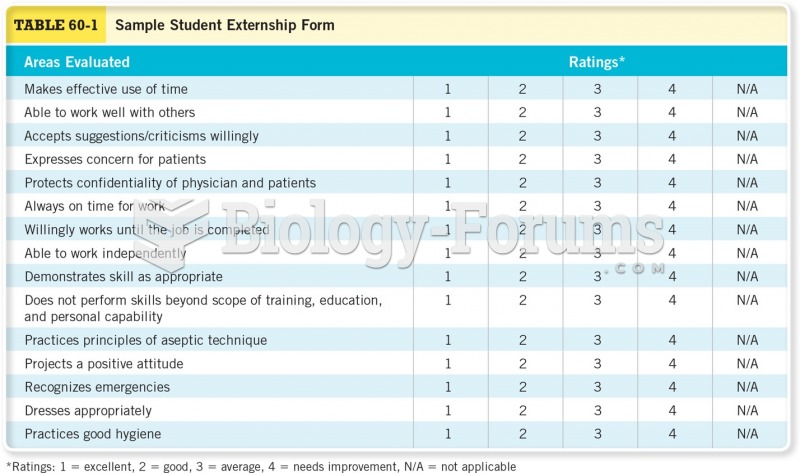
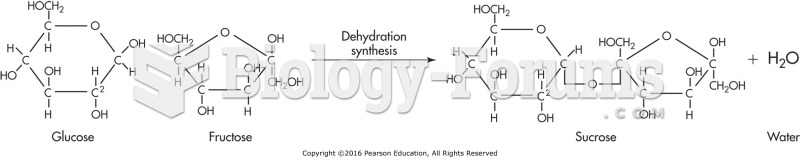
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
HIV testing reach is still limited. An estimated 40% of people with HIV (more than 14 million) remain undiagnosed and do not know their infection status.
Did you know?
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Did you know?
The heart is located in the center of the chest, with part of it tipped slightly so that it taps against the left side of the chest.