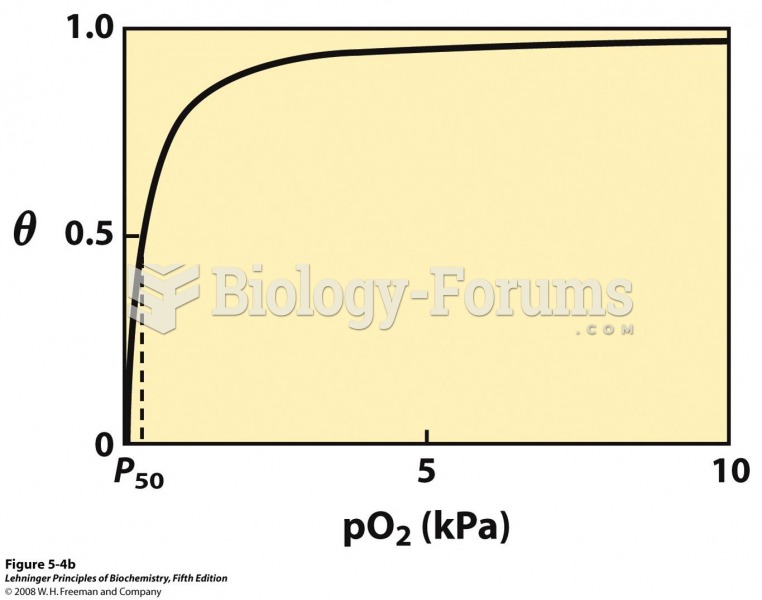
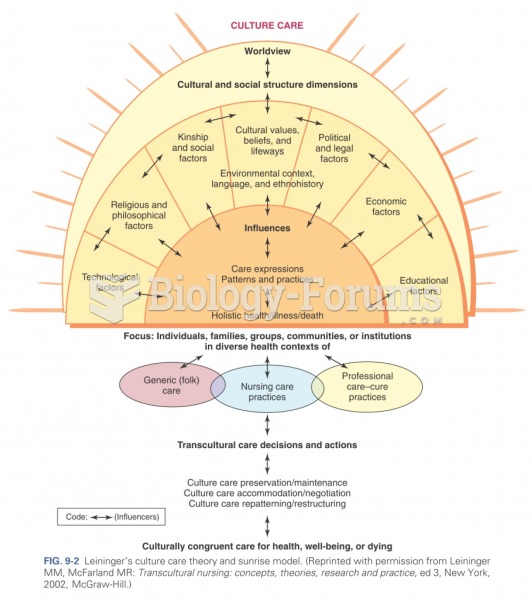
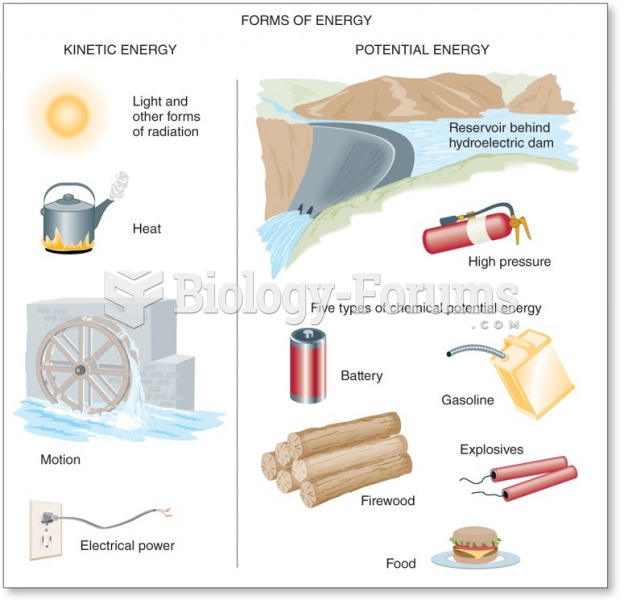
|
|
|
About one in five American adults and teenagers have had a genital herpes infection—and most of them don't know it. People with genital herpes have at least twice the risk of becoming infected with HIV if exposed to it than those people who do not have genital herpes.
Hippocrates noted that blood separates into four differently colored liquids when removed from the body and examined: a pure red liquid mixed with white liquid material with a yellow-colored froth at the top and a black substance that settles underneath; he named these the four humors (for blood, phlegm, yellow bile, and black bile).
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.