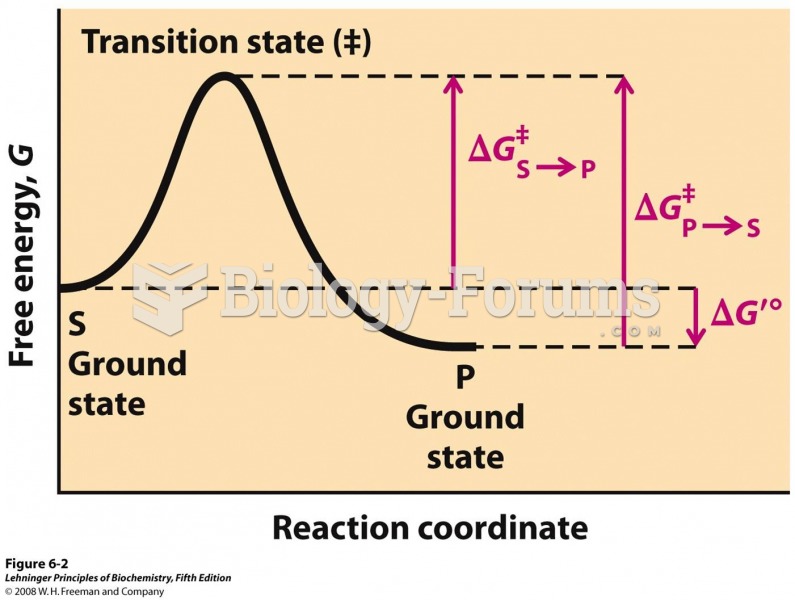
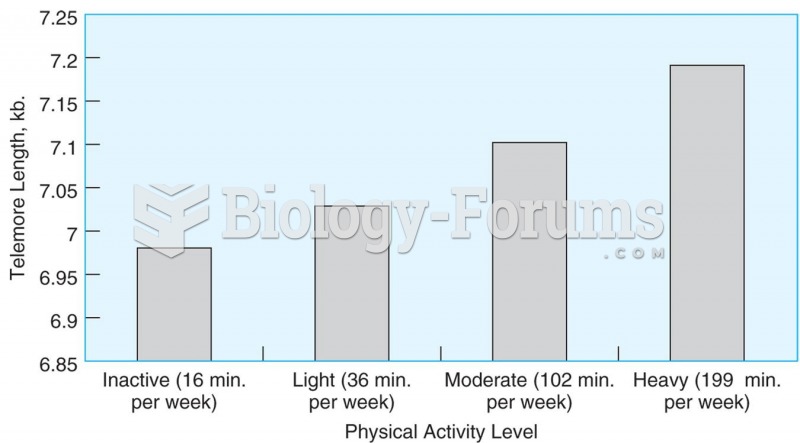
|
|
|
Approximately 500,000 babies are born each year in the United States to teenage mothers.
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.