|
|
|
Oliver Wendell Holmes is credited with introducing the words "anesthesia" and "anesthetic" into the English language in 1846.
Colchicine is a highly poisonous alkaloid originally extracted from a type of saffron plant that is used mainly to treat gout.
All patients with hyperparathyroidism will develop osteoporosis. The parathyroid glands maintain blood calcium within the normal range. All patients with this disease will continue to lose calcium from their bones every day, and there is no way to prevent the development of osteoporosis as a result.
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.
When taking monoamine oxidase inhibitors, people should avoid a variety of foods, which include alcoholic beverages, bean curd, broad (fava) bean pods, cheese, fish, ginseng, protein extracts, meat, sauerkraut, shrimp paste, soups, and yeast.
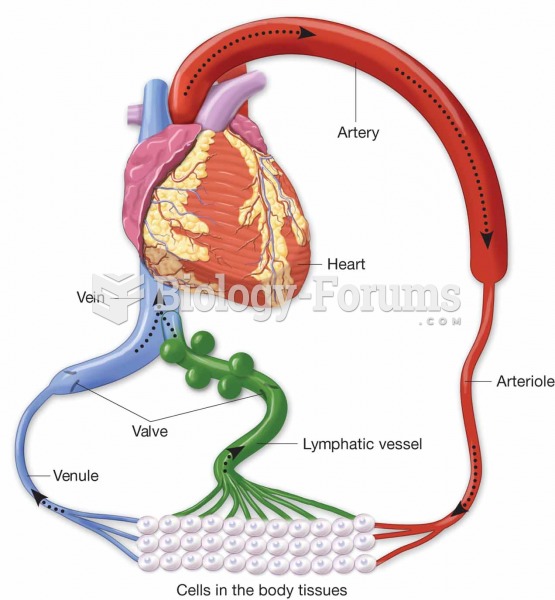 Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t
Lymphatic vessels pick up excess tissue fluid, purify it in the lymph nodes, and then return it to t
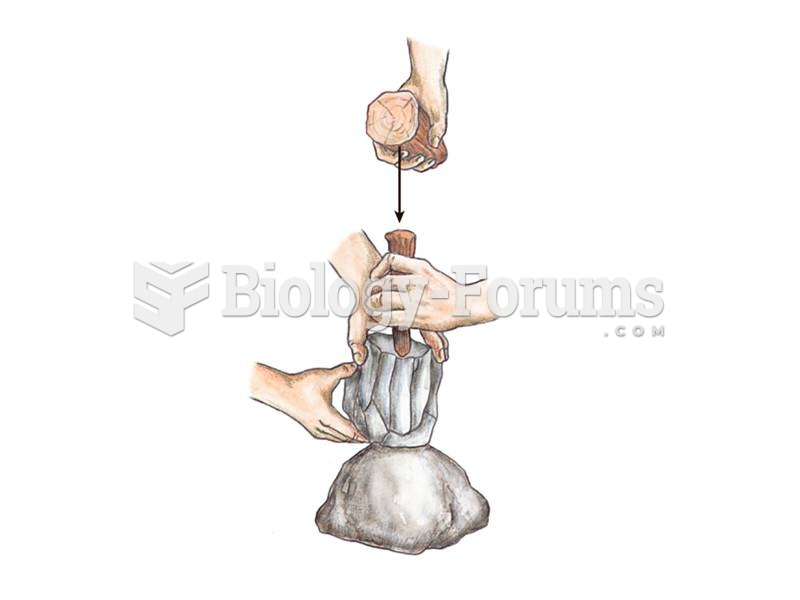 Upper Paleolithic stone tools include blade-based tools as seen here being produced from a blade cor
Upper Paleolithic stone tools include blade-based tools as seen here being produced from a blade cor