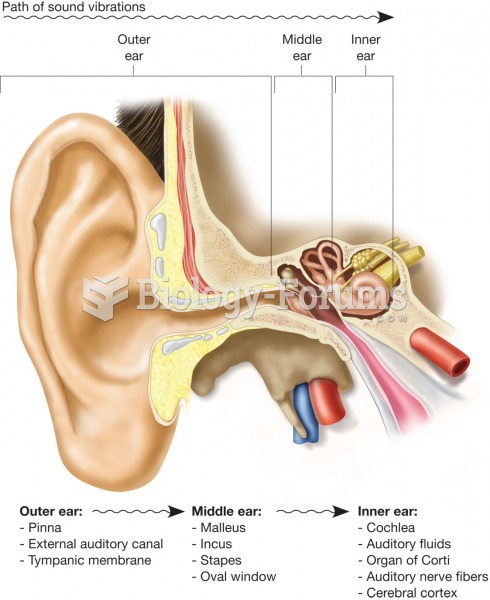
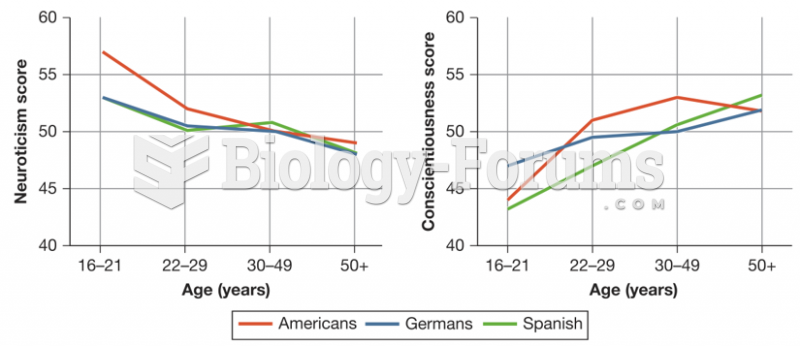
|
|
|
The longest a person has survived after a heart transplant is 24 years.
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.
Drug abusers experience the following scenario: The pleasure given by their drug (or drugs) of choice is so strong that it is difficult to eradicate even after years of staying away from the substances involved. Certain triggers may cause a drug abuser to relapse. Research shows that long-term drug abuse results in significant changes in brain function that persist long after an individual stops using drugs. It is most important to realize that the same is true of not just illegal substances but alcohol and tobacco as well.
Blood is approximately twice as thick as water because of the cells and other components found in it.
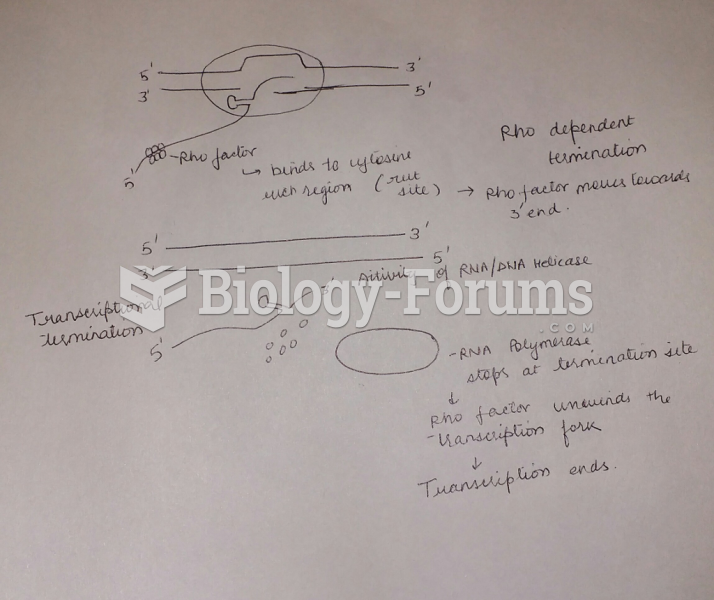 Draw and label a diagram and explain how a factor-dependent transcription (rho-dependent) terminator
Draw and label a diagram and explain how a factor-dependent transcription (rho-dependent) terminator
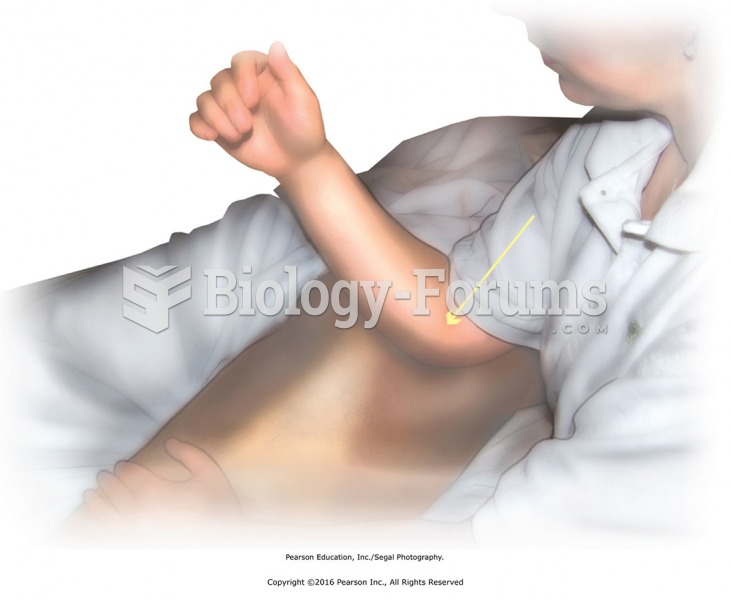 Direct pressure to deep rotator muscles using the elbow. Use the elbow tip to apply pressure moving ...
Direct pressure to deep rotator muscles using the elbow. Use the elbow tip to apply pressure moving ...