|
|
|
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
The ratio of hydrogen atoms to oxygen in water (H2O) is 2:1.
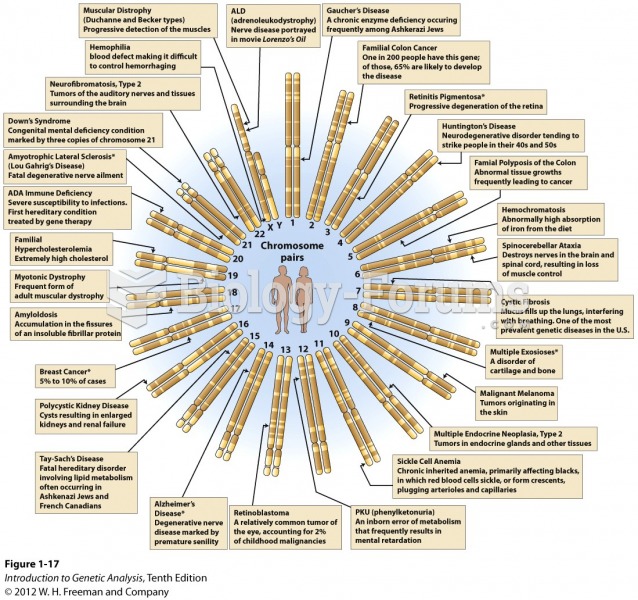
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
Approximately 15–25% of recognized pregnancies end in miscarriage. However, many miscarriages often occur before a woman even knows she is pregnant.
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.