|
|
|
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Certain topical medications such as clotrimazole and betamethasone are not approved for use in children younger than 12 years of age. They must be used very cautiously, as directed by a doctor, to treat any child. Children have a much greater response to topical steroid medications.
Today, nearly 8 out of 10 pregnant women living with HIV (about 1.1 million), receive antiretrovirals.
Human kidneys will clean about 1 million gallons of blood in an average lifetime.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
 An overall view of the four-cylinder engine that is due for a scheduled valve adjustment according ...
An overall view of the four-cylinder engine that is due for a scheduled valve adjustment according ...
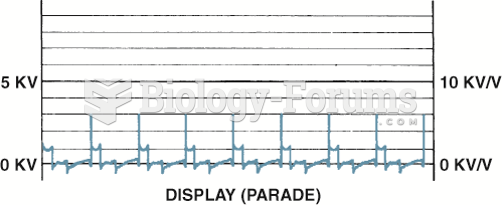 Display is the only position to view the firing lines of all cylinders. Cylinder 1 is displayed on ...
Display is the only position to view the firing lines of all cylinders. Cylinder 1 is displayed on ...