|
|
|
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
For pediatric patients, intravenous fluids are the most commonly cited products involved in medication errors that are reported to the USP.
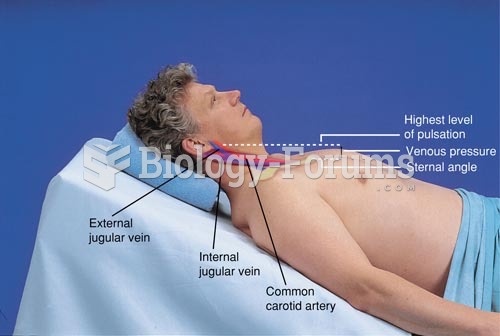
The longest a person has survived after a heart transplant is 24 years.
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.