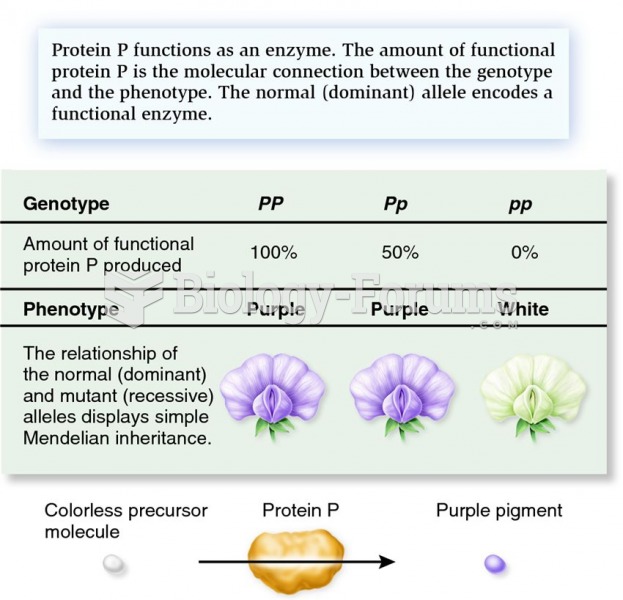
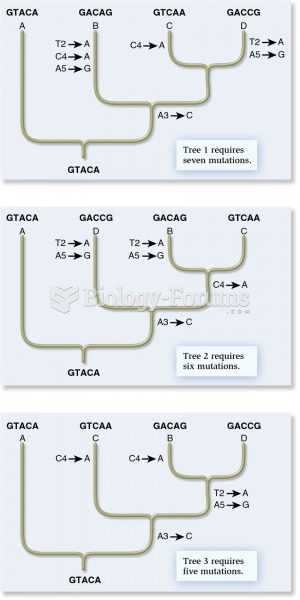
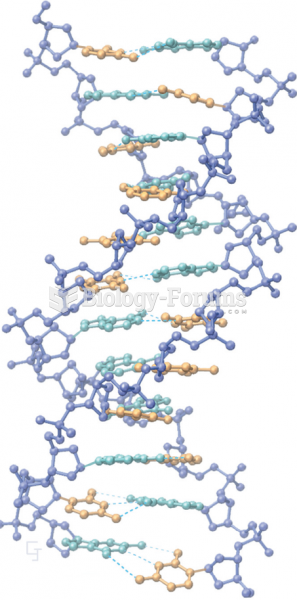
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
Did you know?
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.