|
|
|
Egg cells are about the size of a grain of sand. They are formed inside of a female's ovaries before she is even born.
Stevens-Johnson syndrome and Toxic Epidermal Necrolysis syndrome are life-threatening reactions that can result in death. Complications include permanent blindness, dry-eye syndrome, lung damage, photophobia, asthma, chronic obstructive pulmonary disease, permanent loss of nail beds, scarring of mucous membranes, arthritis, and chronic fatigue syndrome. Many patients' pores scar shut, causing them to retain heat.
A serious new warning has been established for pregnant women against taking ACE inhibitors during pregnancy. In the study, the risk of major birth defects in children whose mothers took ACE inhibitors during the first trimester was nearly three times higher than in children whose mothers didn't take ACE inhibitors. Physicians can prescribe alternative medications for pregnant women who have symptoms of high blood pressure.
Medication errors are three times higher among children and infants than with adults.
Asthma attacks and symptoms usually get started by specific triggers (such as viruses, allergies, gases, and air particles). You should talk to your doctor about these triggers and find ways to avoid or get rid of them.
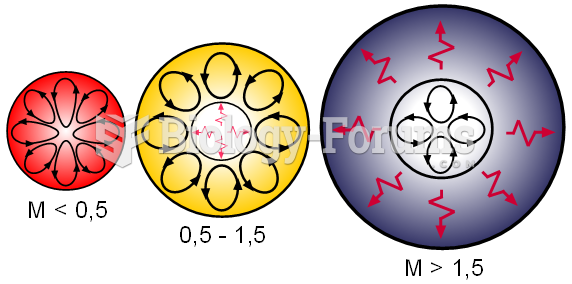 Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
Internal structures of main sequence stars, convection zones with arrowed cycles and radiative zones
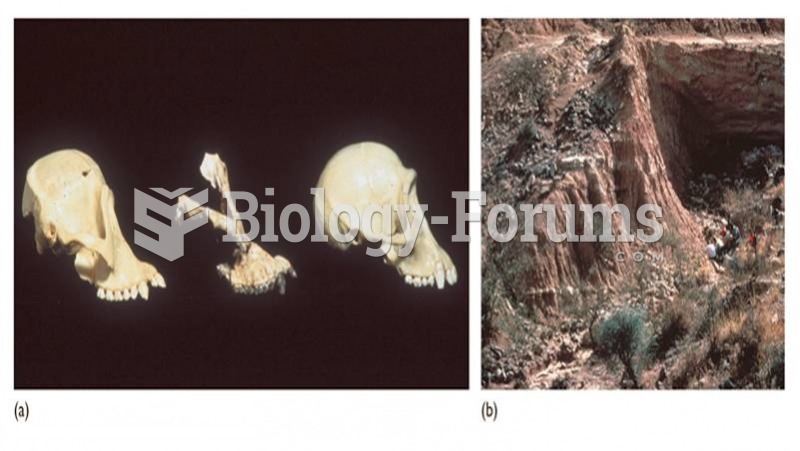 Sivapithecus is a Miocene ape (middle) with anatomical similarities to orangutans (left) rather than
Sivapithecus is a Miocene ape (middle) with anatomical similarities to orangutans (left) rather than