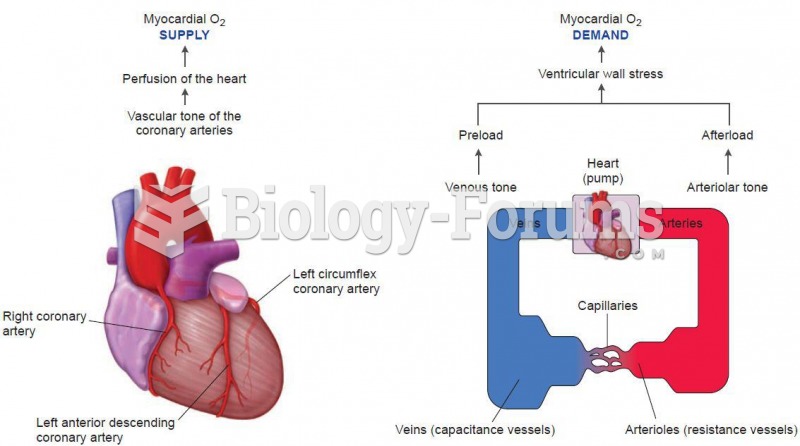
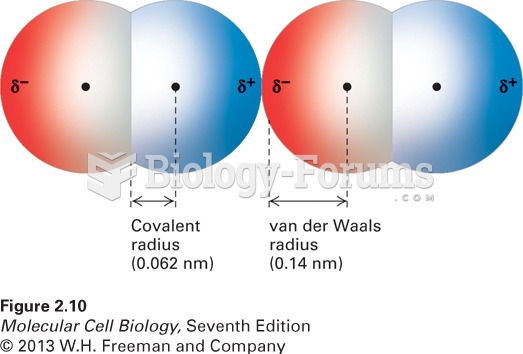
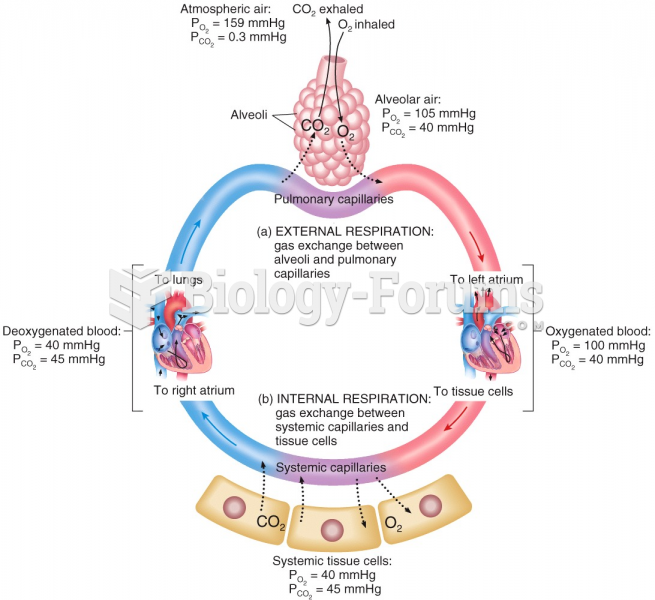
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The strongest synthetic topical retinoid drug available, tazarotene, is used to treat sun-damaged skin, acne, and psoriasis.
Did you know?
Giardia is one of the most common intestinal parasites worldwide, and infects up to 20% of the world population, mostly in poorer countries with inadequate sanitation. Infections are most common in children, though chronic Giardia is more common in adults.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
Critical care patients are twice as likely to receive the wrong medication. Of these errors, 20% are life-threatening, and 42% require additional life-sustaining treatments.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.