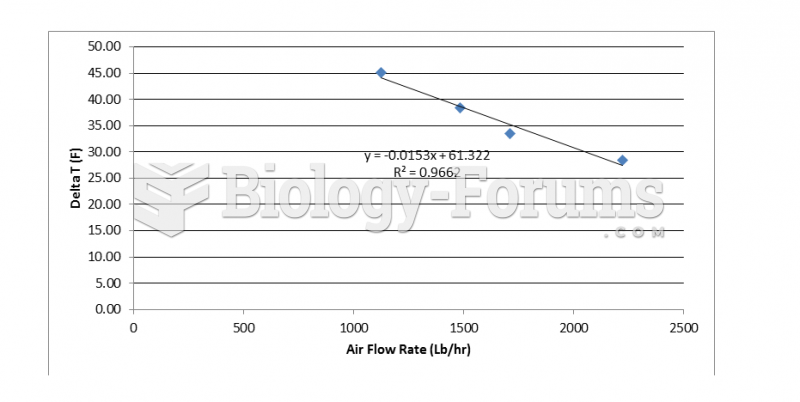
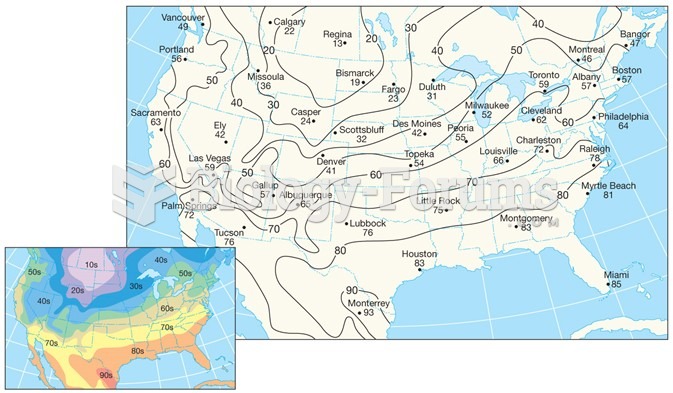
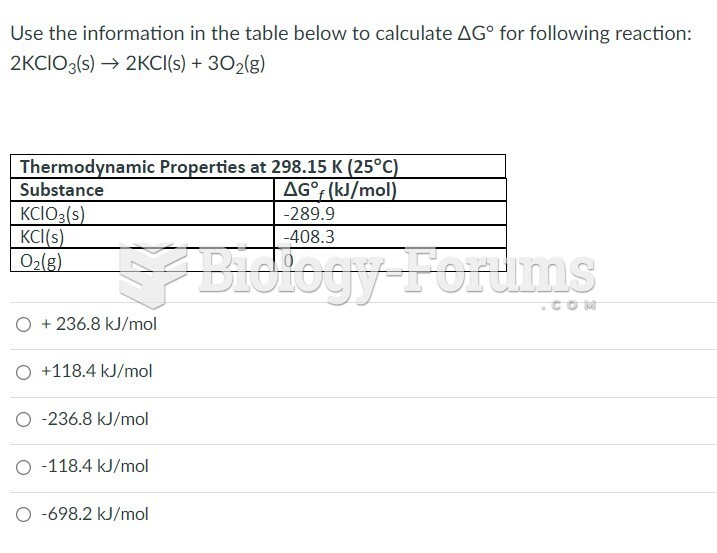
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
Medication errors are three times higher among children and infants than with adults.
Did you know?
Glaucoma is a leading cause of blindness. As of yet, there is no cure. Everyone is at risk, and there may be no warning signs. It is six to eight times more common in African Americans than in whites. The best and most effective way to detect glaucoma is to receive a dilated eye examination.
Did you know?
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.